Home, Search, Index, Links, Pathology, Molecules, Syndromes,
Muscle, NMJ, Nerve, Spinal, Ataxia, Antibody & Biopsy, Patient Info
|
Home, Search, Index, Links, Pathology, Molecules, Syndromes, Muscle, NMJ, Nerve, Spinal, Ataxia, Antibody & Biopsy, Patient Info |
|
Antibodies CK: Serum Electrodiagnostic Neoplasm associations Other systemic Pain Pathology Prognosis Skin lesions Treatment Weakness |
| Skin Involvement 74 |
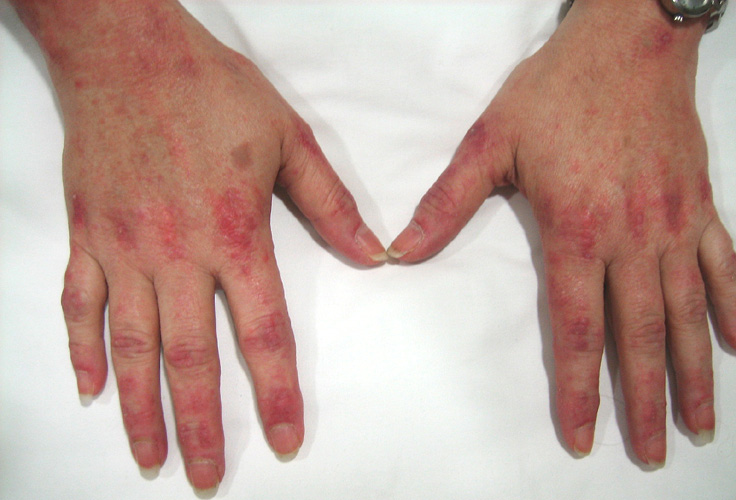 From: M Al-Lozi DM-VP: Rash |
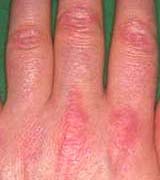 Rash: Erythema From: Chinju, South Korea 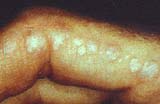 Gottron's papules 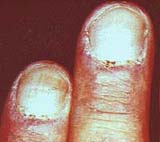 Nailfold lesions |
|
General Antibodies Dermatomyositis Syndromes Neoplasms Pathology Muscle Skin Polymyositis Risk Screening |
|
||||||||||||||||||||||||
|
Classification Inflammation Muscle fibers Necrosis Capillaries Mitochondrial Connective tissue Matrix metalloproteinase MHC-I |
Endomysial inflammation
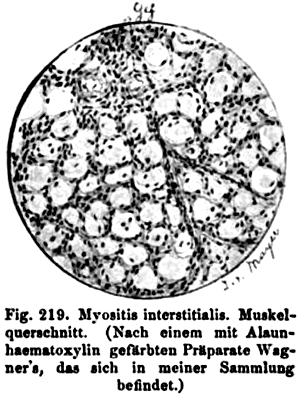 Oppenheim 1894 |
|
Subtypes Clinical features Laboratory Treatment |
| Syndrome | Distinctive features |
| Perimysial pathology (IMPP) |
Interstitial pneumonitis; Raynauds; Arthritis Graft-vs-host disease Aldolase selectively high Antibodies: Jo-1; PL-7, PL-12, t-RNA synthetase |
| Signal recognition particle antibody HMGCR antibody Immune polymyopathy |
Acute onset;
Severe weakness Serum CK: Very high Necrosis, Fibrosis ± Capillary pathology |
| Brachio-Cervical | Brachiocervical weakness B-cell inflammation |
| Histiocytic myopathies | Sarcoid; Immune; Infections Myopathy; Neuropathy; Lung disease Granulomas |
| Regional Ischemic | Rapid onset;
Older patients Necrotic muscle fibers: Many |
|
IIM + VAMP (Vacuoles, Aggregates or Mito Path) Inclusion body myositis (IBM) Mitochondrial (P-COX; PM-Mito) |
Quadriceps & Finger flexor weakness; Older onset age; Steroid resistant Endomysial inflammation; COX- muscle fibers Inclusions; Aggregates; Vacuoles |
| Dermatomyositis | Weakness; Skin rash Perifascicular pathology Muscle fiber atrophy; COX reduced; Capillary pathology |
| Drug-induced | D-penicillamine ICI |
| Other systemic disorders | HIV; Fasciitis |
| Idiopathic | Proximal weakness; High CK; Inflammatory myopathy |
| Collagen vascular disease | Myalgias; Younger onset Scleroderma & Mixed connective tissue disease |
| MAS antibody | Acute onset; Rhabdomyolysis |
| Familial | Homozygosity at HLA-DQA1 locus |
|
|
|
|
|
|
||||
|
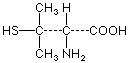
Aromatase inhibitors cGvHD Checkpoint inhibitor D-penicillamine Hydralazine Interferon-α Ipecac Minocycline Osimertinib Other Phenytoin Procainamide TNF-α Toxic oil Also see: Toxic |
|
| D-penicillamine |
|
General Myopathy Neuropathy Pathology |
|
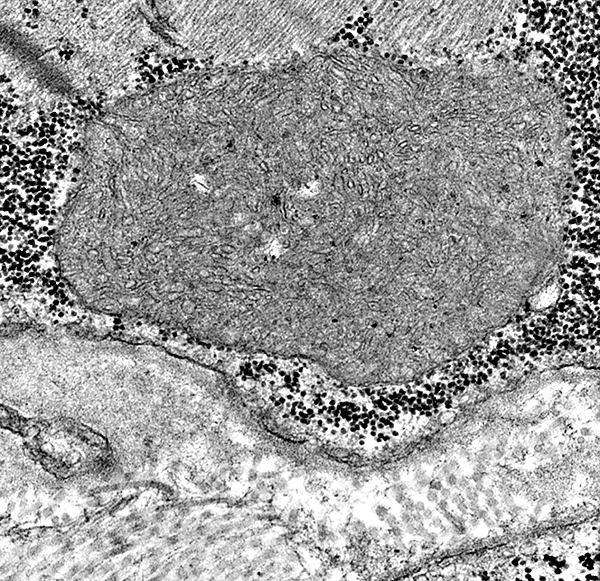 From: O Ni |
MYOPATHIES + SKIN DISORDERS, IMMUNE
|
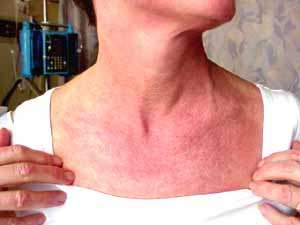 Tim Miller |
|
Child DM-VP Clinical Epidemiology Laboratory Pathology Treatment |
Adult DM-VP variants NXP2 antibody Tif1-γ antibody Child DM-VP variants Tif1-γ antibody NXP2 antibody |
First use of term "Dermatomyositis"
|
|
Full article 1891 Unverricht's first description of a case of Dermatomyositis: Polymyositis acuta progressive. Z Klin Med 1887;12:553 |
|
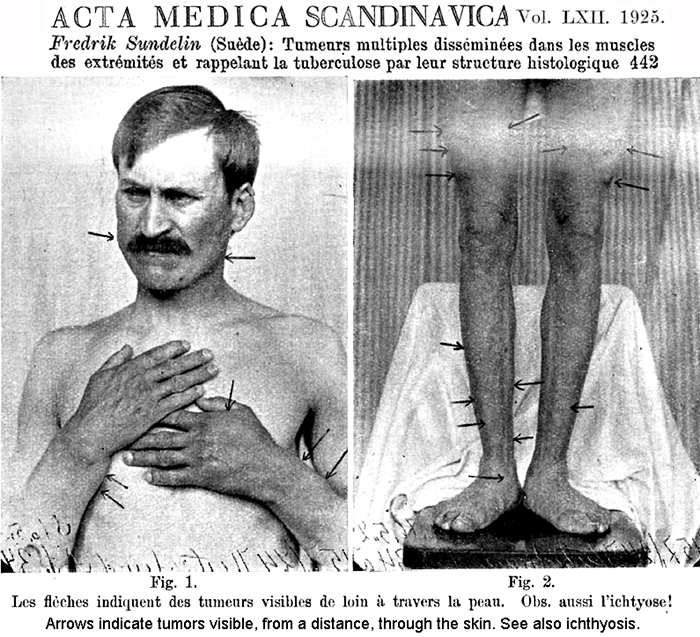
Subtypes Inclusion body myositis (IBM) Antibodies Clinical Epidemiology Laboratory Muscle Pathology Inflammatory myopathy + Mitochondrial pathology (IM-Mito) Muscle pathology |
|
|
|
||||||
|
Absent chondroitin sulfate C Experimental autoimmune myositis Fasciitis Focal myositis Granulomatous Hemophagocytic lymphohistiocytosis Hereditary Infection BACM Influenza Lyme Necrotizing with pipestem capillaries Orbital Perimyositis |
|
|
|
General features Clinical NM syndromes Myopathy Acute Chronic Nodular Neuropathy Sensory Mononeuropathies VII Multiple Mononeuropathy Vasculitis Myelopathy Laboratory Pathology Histopathology Treatment |
|
|
|
|
|
||||
|
|
||||
|
|
|
|
|
|