- History: 1st case reported in pre-Mosaic Eshmuna Code of Babylon, 23rd century B.C
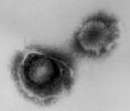
- Virus
- Rhabdovirus: Lyssavirus; Single stranded RNA
- Tissue receptors
- Canine rabies virus: Viral glycoprotein may bind to nicotinic AChRs on muscle
- Bat lyssaviruses: Virus may bind to unknown receptors in the epidermis or dermis
- Eclipse (Latent) phase: Incubation period
- Rabies-virus antigen & genome may persist up to 2 months after inoculation into muscle
- Location: Muscle; Cells at NMJ
- Replication
- In muscle or neural cells
- Some bat related viruses replicate in epidermis & fibroblasts: May be portal of entry
- Further replication after axonal transport to dorsal root ganglia
- Virulence: Related to Glycoprotein in viral envelope

- Argine or lysine at position 333 confer virulence
- Spread to CNS
- No viremia
- Transported to CNS via retrograde axonal transport
- Entry into nerves at NMJs
- May occur with or without replication in peripheral cells
- Transport rate: Rapid; 8 to 20 mm/day
- Interaction between microtubule dynein light chain & viral capsid P protein
- Only neurons affected
- Dissemination within CNS
- Via: Plasma-membrane budding & direct cell-to-cell transmission, or by trans-synaptic propagation
- G protein is required for attachment to neuronal receptors and for trans-synaptic spread
- Preferential localization: Brainstem, Thalamus, Basal ganglia, Spinal cord
- Spread from CNS: Virus may spread centrifugally along nerves
- Unmyelinated axons
- To salivary & serous glands of tongue, heart & skin
- Disease Pathophysiology: ? Associated with immune attack on peripheral nerve or CNS
- Encephalitic rabies & earlier death
- T-cell immunity to rabies virus
- High concentrations of serum IL-2 receptor and IL-6
- Paralytic rabies & longer survival
- Less T-cell immunity
- Lower serum IL-2 receptor and IL-6
- Amount of virus in CNS not strongly related to disease
- Epidemiology
- Endemic in most continents
- Australia with recently identified variant in fruit bats
- None in Antarctica or UK
- Animal Reservoirs
- Only mammals; Non-immunized dogs; Wild carnivores; Bats
- Eastern US: Raccoon
- Mid-Western US: Skunk
- Southwest US: Fox & Coyote
- Western US: Skunk
- Transmission
- Animal bites
- Most common route
- Overall risk: 5% to 80%
- Highest risk of disease & increased severity with bites to head & face
- Lowest risk of disease with bites to lower extremity
- Human cases most commonly from Bats & Dogs
- Thailand: Mostly dog bites
- US: Bat rabies virus most common
- Other
- Scratches: 50x lower risk (0.1% to 1%) than bite
- Aerosol: Occasionally from caves with large bat populations; Laboratory accident
- Tissue transplantation: Corneas
- Transplacental: Rare
- Prevalence
- Worldwide: 50,000 deaths per year; Most in India from dog bites
- US: 1 or 2 human cases & 8,000 animal cases per year
Clinical
- Incubation period
- Average 1 to 2 months; Range of 1 week to 6 years
- Bite factors
- Site of bite: Decreased incubation with more Proximity to the CNS
- Severity of bite
- Viral load in bite
- Longest incubation periods with Australian bat lyssavirus
- Short incubation periods (< 1 week)
- Direct inoculation of virus into nervous tissue
- Example: Brachial-plexus injury from dog bites
- Patient factors: Age; Immune status
- Chloroquine treatment may increase likelihood & severity of rabies
- Prodrome
- General symptoms: Headache; Fever; Hyperactivity
- Sensory
- Paresthesias & Pain
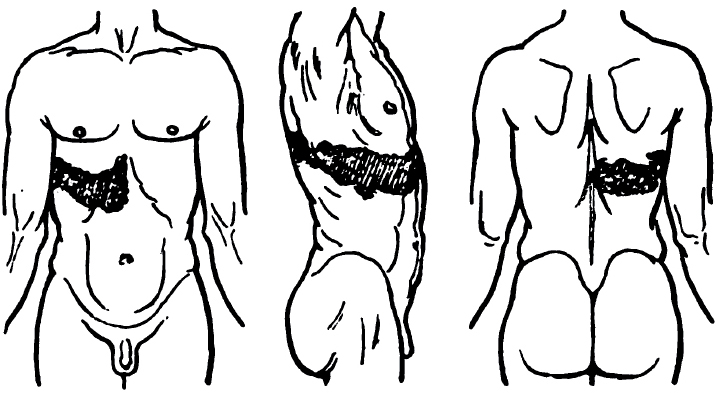
- Especially at inoculation site spreading over limb
- Sensations: Burning, numbness, tingling, itching, or pruritus
- Duration: Few days to week
- Frequency: Bat bite (75%); Dog related (33%)
- Virus moves from periphery to dorsal root ganglion
- Death often occurs in next 2 weeks
- No loss of sensation
- Weakness or Spasms: One extremity
- Encephalopathy (Furious cases): Neurological signs, then coma
- Frequency: Occurs in 2/3 of patients with classic rabies
- Clinical
- Hyperactivity: Hydrophobia or aerophobia; Early in course
- Spasms
- Location: Neck, diaphragm & pharyngeal muscles
- Triggered by sensory stimuli
- Development of drowsiness & coma: Reduced frequency of typical spasms
- Inspiratory spasms: May occur without stimulation; May persist during coma
- Gag reflex: Increased
- Encephalopathy
- Agitation; Hallucinations; Myoclonus; Seizures (Late)
- Mentation: Fluctuations; May be normal early in course
- Autonomic hyperactivity
- Fever: May be very high; Common
- Hyperhidrosis
- Salivation: Increased
- Pupils: Poorly reactive; Dilated; May be transient or asymmetric changes
- Other: Piloerection; Pulmonary edema; Priapism & Spontaneous ejaculation
- Paralysis: May occur late in course
- Course: Progressive
- Coma phase
- Inspiratory spasms may occur
- Cardiac: Sinus tachycardia; Nodal rhythms; Reduced ejection fraction
- Hematemesis (50%): Pre-terminal
- Death
- Time: In < 7 days from onset
- Due to: Respiratory or Circulatory insufficiency
- Recovery
- Rare
- Often with atypical presentation
- Early prophylaxis with cell-culture vaccine
- High concentrations of neutralizing antibodies
- Improvement over months
- Chronic sequellae common
- Paralytic (Dumb; Myelitic) rabies: 20% of patients
- General: Little hyperactivity early in course; Fever common
- Weakness
- Onset in bitten extremity
- Proximal > Distal
- Progression: Quadriplegia; May involve bulbar & respiratory muscles
- Percussion myoedema: Chest; Deltoid; Thigh
- Cranial nerve
- Pharyngeal
- Facial: May be unilateral early; Often becomes bilateral
- Sensory: Paresthesias; No loss of sensation
- Tendon reflexes: Absent
- Autonomic: Piloerection
- Phobic spasms: 50%
- Urinary incontinence
- Symptoms of furious rabies
- Mild; Late-appearing
- Inspiratory spasms: Pre-terminal
- More CSF cellularity
- Course
- More prolonged than encephalitic rabies
- Death: May be due to respiratory failure; After ~13 days
- Variant syndrome
- Associations: Most common after insectiverous bat bite; Some dog-related
- Local limb prodromes especially common
- Local neuropathic pain
- Choreiform movements of bitten limb during prodrome
- Often evolves to furious rabies
- Sensory: Objective loss may occur
- Face weakness
- CNS
- Occasional brainstem features: Anisocoria; Ptosis; Diplopia; Nystagmus; Myoclonus
- No phobic spasms or autonomic hyperactivity
- Mortality
- Reduced by treatment
- Dog bites: 38% to 57%; Depends on severity + location of wound & saliva virus concentration
- Other species (wolves): Up to 80%
- Higher with: Bites on head, face, neck & hand; Bleeding
- Superficial bat bites: Risk due to viral replication in epidermis & dermis
Diagnosis
- Inoculation of mice with patient saliva
- Rabies antigen in skin within hairline
- Saliva PCR
- Antibodies (Serum & CSF)
- May only occur late in course
- Titers high in non-immunized patient
- Very high titers in previously immunized patient
- Evaluate animal for rabies: Confinement or Pathological examination
- Fluorescent examination of brain for rabies antigen: High sensitivity
- MRI: Increased T2 signal in brainstem, hippocampi, hypothalami, white matter & cortical grey
Pathology
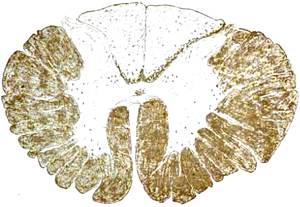
- Distribution: Encephalitis & Myelitis
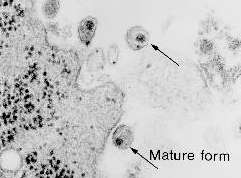
- Cellular: Cytoplasmic eosinophilic inclusion bodies (Negri bodies) in neurons
- External link: CDC
Treatment & Prophylaxis
- Wound care
- Rabies vaccine
- Use duck embryo or tissue culture prepared vaccines
- Course of treatment
- Neutralizing antibody should be maintained for 1 year
- 5th dose at 30 days necessary
- Vaccines prepared in brain or spinal cord may produce
- Uses
- Post-exposure
- Prophylactic in high risk groups (Veterinarians): Only 1 or 2 booster doses needed after exposure
- Experimental: Vaccination of wildlife using recombinant vaccinia vaccine (live) in animal bait
- Rabies immunoglobulin
- Skin test first if using equine form
- Indications: Transdermal bites; Licks over mucosa
- After disease onset: Symptomatic
External link: CDC
|

CDC
Rabies virus
|
|