Botulinum Toxin: Proteins & Transport
- General: Botulinum toxins
- Production
- Only when the spores germinate
- Germination circumstances
- Anaerobic conditions
- Low acidity (pH > 4.5)
- Low salt & sugar content,
- Temperatures
- 37°F–99°F (3°C–37°C)
- Varies with serotype
- Most strains produce single serotype
- Molecular
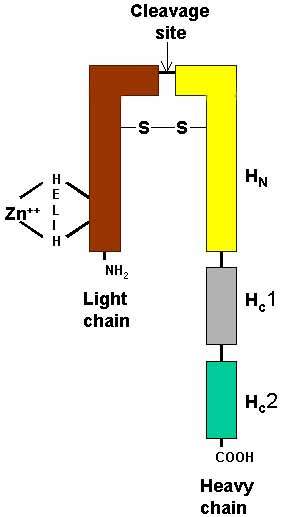
- Zinc-endopeptidase protein
- Produced as
- Single chain protein (protoxin)
- Molecular weight ~150 kDa
- Types share common domain organization
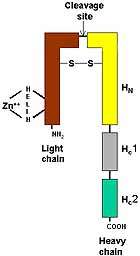
- Processed into mature toxins by cleavage into 2 subunits
- 50 kDa light chain (LC)
- 100 kDa heavy chain (HC)
- Subunits remain connected through a conserved disulfide bond
- Disulfide bond is not present in BoNT/Wo
- Released from bacteria as part of noncovalent multimeric complex
- Auxiliary proteins
- Hemagglutinins (HA)

- Nontoxin, nonhemagglutinin (NTNH)


- Complex with botulinum
- May protect toxin at low pH in GI tract
- Dissociates spontaneously at physiologic pH
- Some components necessary for toxicity
- No direct role of auxiliary proteins in toxin-induced Ach blockade
- Neurotoxic types of botulinum toxin
-
A
 ,
A2 ,
A2
 ,
B ,
B
 ,
C1 ,
C1
 ,
D ,
D
 ,
E ,
E
 ,
F ,
F
 ,
G ,
G

- Type A: Most severe disease & frequent need for ventialtory support
- Type B: Milder disease
- Types C & D: Not reported in recent outbreaks
- Type D: May not be absorbed from human GI tract
- Type E: Foods of aquatic origin; Varied disease severity; GI symptoms common
- Type F: Rapid progression & earlier rocovery
- Tetanus toxin: Sequence homology 30% to 40%
- Cleavage of Botulinum protoxins: Into 2 chains
- Heavy (100 kDa) chain
- C-terminal region (HC) of Heavy (H) chain: Binds to surface of target nerve cells
- N-terminal region (HN) of H chain: Translocates L chain across membranes
- Light (L) chain (50 kDa)
- Light chains have a tetrahedral zinc binding motif: Contains
- Consensus HELIH amino acid sequence
- 2 histidines, glutamate & water molecule
- Structure resembling thermolysin-like endoproteases
- Contain toxic activity
- Zinc-dependent endopeptidase
- Cleave proteins forming synaptic vesicle docking & fusion complex
- 3-D Geometry: Catalytic sites buried deeply within protein
- Heavy & Light subunits linked by disulfide bond
- Double chain product is active form: Inhibits cholinergic transmission
- Protease producing cleavage
- Often contained by producing organism
- May also occur in GI tract
- Toxin passes from GI tract to vasculature: Mechanisms
- Transport cell: Absorptive enterocyte
- Binding to the apical surface of epithelial cells
- Receptor-mediated endocytosis
- Transcytosis through cells
- Delivery to the basolateral surface of cells
- Ability of toxin to cross cells depends on
- Toxin type
- Able to cross cells: Botulinum type A & B
- Not able to cross cells: Botulinum type C & Tetanus
- Heavy chain of botulinum toxin: C-terminus
- Toxin passes out of vasculature to presynaptic regions
- ? Mechanism
- Does not cross blood-brain barrier
- Other absorption routes
- Botulinum toxin may be absorbed from respiratory system
- General action: Blocks exocytosis
- Most potent action: Blockade of cholinergic terminals
- Other blockade of exocytosis: At higher concentrations of toxin
- Other nerve terminals: Norepinephrine, Serotonin
- Non-neural cells: If membrane receptors present
|
|
Botulinum Toxin
Type B |
|