Arsenic (inorganic)
- Toxicity
- Forms: Toxicity
- Inorganic >; Organic
- As3+ > As5+
- Sources: Suicidal, Homicidal, Occupational, Environmental
- Mining
- Inorganic arsenic: Exposure to by-products of copper & lead smelting
- Ingestion of trivalent arsenic
- Suicide
- Homicide
- Food or water contamination: Endemic regions
- Groundwater contamination
- Locations: West Bengal, India; Bangladesh; Inner Mongolia
- West Bengal, India
- Drinking water: 6 million people exposed
- 300,000 people manifest signs of chronic arsenicosis
- Contaminated opium: Punjab
- Chinese medicinal herbal preparations
- Toxic agent: Arsenic sulfide
- Chronic poisoning
- Marine organisms
- May contain: Trimethylated organoarsenic, Arsenobetaine
- No known toxic effects
- Well-absorbed
- Excreted unchanged in urine
- Chemotherapy: As03
91
- Acute promyelocytic leukemia
- More neurotoxicity with
- Obesity (High BMI > 35; Weight > 100 kg)
- Increased dose (> 500 mg; > 10 mg/day)
- Onset: 2 to 8 weeks after exposure
- Route of intoxication
- Usually oral
- Absorbed via GI tract
- Accumulates in: Liver, Kidney, Lung, Spleen, Aorta, Skin, Hair, Nails
- Metabolism of inorganic arsenic
- Methylation
- Mainly in liver
- To Monomethylarsonic acid & Dimethylarsinic acid
- May be slower in Caucasians
- Excretion: Urine; Along with residual inorganic arsenic
- Pathophysiology: Uncouples oxidative phosphorylation
- Trivalent As+++
- More toxic than pentavalent form
- Interaction with: Thiol on lipoic acid in PDH complex

- ? Inhibits pyruvate → Acetyl CoA reaction: Substitutes for phosphate
- Possible association: Thiamine deficiency
- Clinical syndromes: Dose related
- General: Garlic-like smell to breath & tissue fluids
- Massive overdose: Subacute neuropathy
- Early
- GI: Vomiting & Diarrhea; Probably 2° GI irritation
- Autonomic: Tachycardia & Hypotension; 2° GI fluid loss
- Neuropathy
- Onset
- Delayed: After 10 to 20 days
- Distal paresthesias & numbness
- Evolution
- Over 2 to 5 weeks
- Sensory > Motor
- Large fiber sensory modalities
- Systemic signs: Few
- Pathology: Distal axonal loss ± Demyelination
- Treatment: Chelation with BAL or penicillamine for months
- Recovery: Slow over years
- Lower level exposures: Slowly progressive; 3 stages
- Exposures
- Chronic environmental absorption > 500-1000 μg/d
- Chemotherapy
- Onset (1st stage): Malaise; Anorexia; Vomiting
- 2nd stage
- Membrane irritation
- Skin
- Hyperkeratosis
- Feet & Hands
- Precancerous state
- Arginine variant of codon 72 of p53 gene
- Homozygosity associated with: Keratosis
- Dark (Gray) skin
- Hypopigmentation ("Raindrop pattern")
- Nails: White stria (Mees lines), May occur months after acute episode
- Pitting edema
- Aplastic anemia
- Renal damage
- Polyneuropathy
- Sensory loss: Stocking-glove; Large > Small fiber
- Weakness: Mild
- CSF: Protein mildly increased
- Pathology: Axonal loss; Distal
- Recovery: Common; Better in mildly affected
- More common in patients with skin hyperkeratosis
- Other
- Myopathy: Arsenic trioxide
- Arsine gas: Hemolysis
- Liver
- Cardiac
- CNS: Seizures; Encephalopathy
- Long term: Carcinogen; Lung,
Skin,
Liver, Kidney, Bladder
- Diagnosis: Arsenic levels
- Acute (< 6 weeks): Urine
- Chronic
- Tissues: Hair (Pubic); Fingernails
- Level: > 1μg/g
- False positive urine levels
- 25 to 1,000 μg/24 hours
- 2° to consumption of nontoxic organic arsenates in seafood (shellfish)
- Treatment: Chelation
- BAL (Dimercaprol; IM; 100 mg/ml in peanut oil)
- Mild poisoning: 2.5 mg/kg qid x 2d, bid x 1d, then qd
- Severe poisoning: 3 mg q4h x 3d, qid x 1d, then bid
- D-penicillamine: May produce neurological complications
- ? 2,3-dimercaptopropanesulphate (DMPS): 5 mg/kg q 4 hours
- Treat until urine arsenic < 25 μg/24h
|
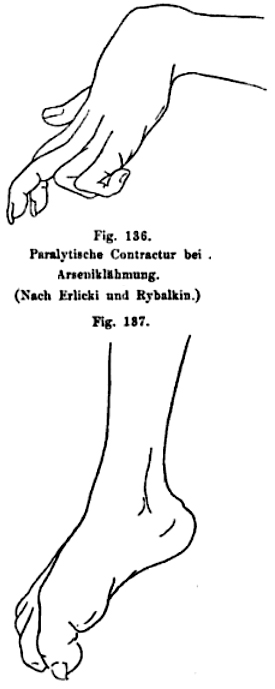
Oppenheim 1894
|
|