Home, Search, Index, Links, Pathology, Molecules, Syndromes,
Muscle, NMJ, Nerve, Spinal, Ataxia, Antibody & Biopsy, Patient Info
|
Home, Search, Index, Links, Pathology, Molecules, Syndromes, Muscle, NMJ, Nerve, Spinal, Ataxia, Antibody & Biopsy, Patient Info |
|
CIPNM Comparative features Treatment strategies CIDP Clinical features Associated disorders Laboratory features Pathology Treatments Variants Antibodies Contactin-1 GALOP syndrome GD1a: Motor-Sensory neuropathy GD1b: CANOMAD; CANDA GM1: Immune Motor Neuropathy Lgi-4 MAG associated neuropathy Neurofascin Sulfatide Neuropathies with Myelin pathology Osteosclerotic Myeloma POEMS Syndrome Antibody tests Also see: Immune axonal neuropathies |
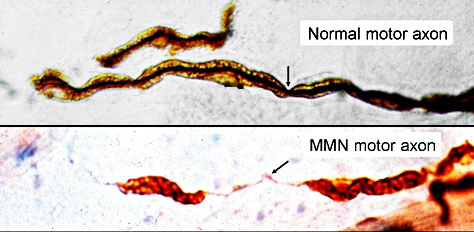 Immune Motor Neuropathy (IMN) Motor Axons: Segmental demyelination |
| Chronic Immune Peripheral Nerve Myelinopathies (CIPNM) COMPARATIVE FEATURES | ||||||
|---|---|---|---|---|---|---|
| Neuropathy (Antibody) |
Clinical Features | Electrophysiology | Antibody | M-Protein* | Myelin Pathology | Treatment |
|
Chronic Immune Demyelinating Polyneuropathy (CIDP) |
Motor > Sensory Weakness: Proximal & Distal Symmetric Onset: 1 to 80 yrs Chronic/Relapsing |
Motor + Sensory Δ NCV: Slow Conduction Block Distal Latency: Long F-waves: Slow |
? | 15% | Segmental Demyelination Onion bulbs |
T-cell immunosuppression Prednisone Cyclosporine A Methotrexate HIG Plasma Exchange |
| Multifocal CIDP Also see: CIDP variants |
Chronic Motor > Sensory Weakness: Distal > Proximal Asymmetric Arms > Legs Onset: 15 to 75 yrs |
Motor + Sensory Δ NCV: Slow Conduction Block Distal Latency: Long F-waves: Slow |
? | ? | Segmental Demyelination, Multifocal Onion bulbs |
T-cell immunosuppression Prednisone HIG |
|
Myelin- Associated Glycoprotein (MAG) |
Sensory > Motor Distal; Symmetric Gait disorder Tremor Onset: > 50 yrs Slowly progressive |
Motor + Sensory Δ Distal Latency: Long NCV: Slow No conduction block Axon Loss: Distal legs |
Target: MAG M-protein: IgM |
90% | Wide Spacing |
B-cell immunosuppression Rituximab Cyclophosphamide ± Plasma Exchange ? Fludarabine Not: HIG or Steroids |
| Polyneuropathy Organomegaly Endocrinopathy M-protein Skin changes (POEMS) |
Sensory & Motor Symmetric Onset: 25 to 60 yrs |
NCV: Slow No conduction block Axon Loss |
Target: ? M-protein IgAλ or IgGλ |
100% | Uncompaction | Local lesions: Irradiation Systemic Chemotherapy Stem cell transplant |
| Myelinopathy + Neurofascin -155 Antibody |
Sensory & Motor Distal Tremor Onset: Adult Progressive |
NCV: Slow Distal latency: Long Conduction block: Some |
Target Neurofascin-155 Class: IgG4 |
No | Nodes wide Redundant myelin |
Rituximab Corticosteroids |
|
Immune Motor Neuropathy (IMN) |
Motor only Distal > Proximal Arms > Legs Asymmetric Onset: 25 to 80 yrs Slowly progressive |
Motor only Conduction Block Axon Loss: Distal EMG: Denervation with disease progression |
Targets GM1 ganglioside NP-9 or NS6S Class: IgM Frequency: 50% |
20% | Segmental Demyelination, Multifocal |
IVIg (Conduction block) B-cell immunosuppression Rituximab Cyclophosphamide ± Plasma Exchange |
| Polyneuropathies with Inconsistent Myelin Pathology | |||||
| Contactin-1 Ab |
Sensory & Motor Distal or Diffuse Onset: Adult, late Progressive |
Distal latency: Long Conduction block |
Target Contactin-1 Class: IgG or IgG4 |
No | Prednisone Not HIG |
| CANDA |
Sensory >; Motor Distal Onset: Adult Tremor Progressive |
Distal latency: Mildly Long SNAP amplitude: Reduced |
Target GD1b Class: IgM |
90% | Rituximab |
| GALOP | Gait Disorder Sensory > Motor Distal; Symmetric Onset: > 50 yrs |
Motor + Sensory Δ Distal Latency: Long NCV: Slow No conduction block |
Target Sulfatide in lipid membrane Class: IgM |
80% | HIG Cyclophosphamide ± Plasma Exchange |
| Sulfatide |
Slowly progressive Sensory > Motor Distal; Symmetric Onset: > 45 yrs |
Distal Latency: Long NCV: Slow Axon Loss: Distal |
Target Sulfatide Class: IgM |
90% | HIG Cyclophosphamide ± Plasma Exchange |
| GM2 & GalNAc-GD1a Ab |
Sensory > Motor Ataxia: Limb & Gait Distal Symmetric or Asymmetric Onset: Adult Slowly progressive |
NCV: Slow |
Targets GM2 GalNAc-GD1a Class: IgM |
Common | HIG |
|
Clinical features Associated disorders Comparative features Laboratory features Pathology Treatments Variants |
|
|
|
|
Antibodies Clinical Electrodiagnostic Epidemiology Laboratory Pathology Treatment Variants & DDx |
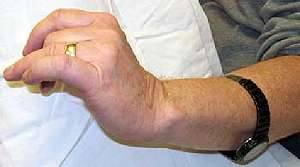 MMN: Focal weakness Finger extension: Involvement varied |
 MMN: Median nerve conduction block Thenar eminence: Weakness No atrophy early in disease |
|
|
|
|
|
|

|
|
|
|
||||
|

|

|
|
|