Inflammatory Myopathies with Vacuoles, Aggregates & Mitochondrial Pathology (IM-VAMP)
Inclusion Body Myositis (Subtype)
|
Pathology features Inflammation Focal invasion of fibers General IBM Immune Cell types Endomysial Perimysial Muscle fibers Aggregates Histochemistry AMPDA Amyloid-like Cytoplasmic bodies Eosinophilic Components αB-crystallin β-Amyloid Desmin LC3 p62 SMI-31 TDP-43 Ubiquitin VCP Ultrastructure Pathology Focal invasion MHC Class-1 Mitochondrial Ultrastructure Myopathy Myonuclei Vacuoles Capillaries Variant syndromes IM-VAMP in HIV PM-Mito Ultrastructure Aggregates Focal invasion by cells Mitochondria Vacuoles |
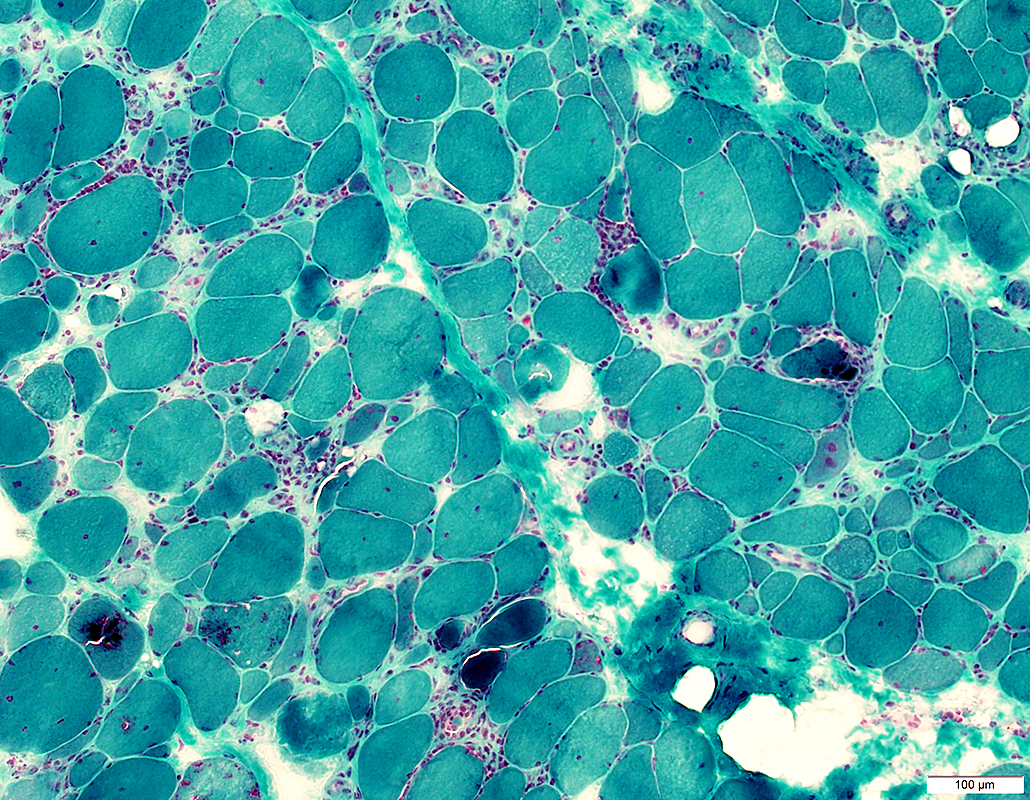 Gomori trichrome stain IBM (IM-VAMP): Myopathy; Inflammation; Aggregates & Vacuoles
|
IM-VAMP (IBM): Muscle Pathology
- General description
- Inflammatory Myopathy with Vacuoles, Aggregates & Mitochondrial Pathology (IM-VAMP)
- Inflammation: Mononuclear cells
- Locations
- Endomysium: Most common
- Also in some patients: Perimysium & Perivascular
- Focal invasion of muscle fibers
- Cell types in foci
- T-cells
- B-cells: CD20
- Usual: Few or none present
- Foci reported: sIBM associated with chronic lymphocytic leukemia 78
- Plasma cells: CD138
- Scattered in foci
- Histiocytes
- Many in: Cell foci; Regions of focal invasion of muscle fibers
- Increased GBP6 (IFN-γ induced)
- Locations
- Muscle fibers
- Myopathic changes
- Varied muscle fiber size
- Regenerating muscle fibers
- Groups of small fibers
- Endomysial connective tissue: Variably increased or normal
- Muscle fiber hypertrophy: More common than in other immune myopathies
- MHC-I
- Expression: On surface, ± cytoplasm, of most muscle fibers
- Occurs on morphologically normal & abnormal muscle fibers
- No specific association with inflammatory cells or foci
- Rimmed vacuoles with granular material & filaments
- Histochemistry: Best visualized with Congo Red stain
- Contain
- Filaments: 15 to 18 nm
- Several proteins β-Amyloid, Desmin; Ubiquitin; Transglutaminases 1 & 2
- Aggregates & Related proteins
- Protein aggregates: May contain
- Cadherin I
 4
4
- Present in cytoplasm of some muscle fibers (68% of IBM)
- Associated with increased SQSTM1 in muscle fiber cytoplasm
- Anatomy: Aggregates often not related to vacuoles
- Ultrastructure
- Also see: Cytoplasmic bodies
- Mitochondrial disorders
- Pathology location: Muscle fiber mitochondrial Δ
- Segmental: 75 μm to > 1 mm along fiber length
- Fiber distribution: Scattered in muscle
- COX- muscle fibers
- Frequency: Up to 15% of muscle fibers
- MT-ND4 (major arc locus): Reduced
- MT-ND2 (minor arc locus): Normal
- mtDNA Deletions & Duplications: Present in 85% of fibers
- Oxidative enzyme activities: Combined complex I & IV deficiency
- Respiratory chain-deficient fibers: May be smaller than other fibers
- No association: Invasion of inflammatory cells or rimmed vacuoles
- Syndromes
- SDH+ muscle fibers (Mitochondrial proliferation)
- mtDNA disorders: IBM muscle fibers
3,
92
- Copy number in IBM: 42% of control
- Deletions: Multiple in > 50% of IBM
- Deletions & Duplications in muscle
- IBM: Mean heteroplasmy level of 10% (Range 1%-35%)
- Controls: Mean heteroplasmy level of 1% (Range 0.2%-3%)
- Variations in deletions: Common
- Single muscle fiber: May have multiple different deletions
- Different muscle fibers: Have different sizes & locations
- Patterns in IBM
- Most deletions: Located in major arc of mtDNA; Involve ND4
- Unusually large deletions (10%): Involve ND1 & ND4
- mtDNA regions: m.534-4,429, m.6,330-13,993, m.8,636-16,072
- Regions may be flanked by: Repetitive sequences
- Similar regions to: Mutations in controls & POLG1 mutations
- Breakpoint hot spots
- 5' end: tRNALeu gene (np3230–np3304)
- 3' end: np16070
- Other: ~np13923; ~np12300
- Deletions & Duplications in muscle
- Duplications: Present in many sIBM patients
- Single nucleotide variants: More in IBM than age-matched controls
- Ultrastructure
- Pathology location: Muscle fiber mitochondrial Δ
- Myopathic changes
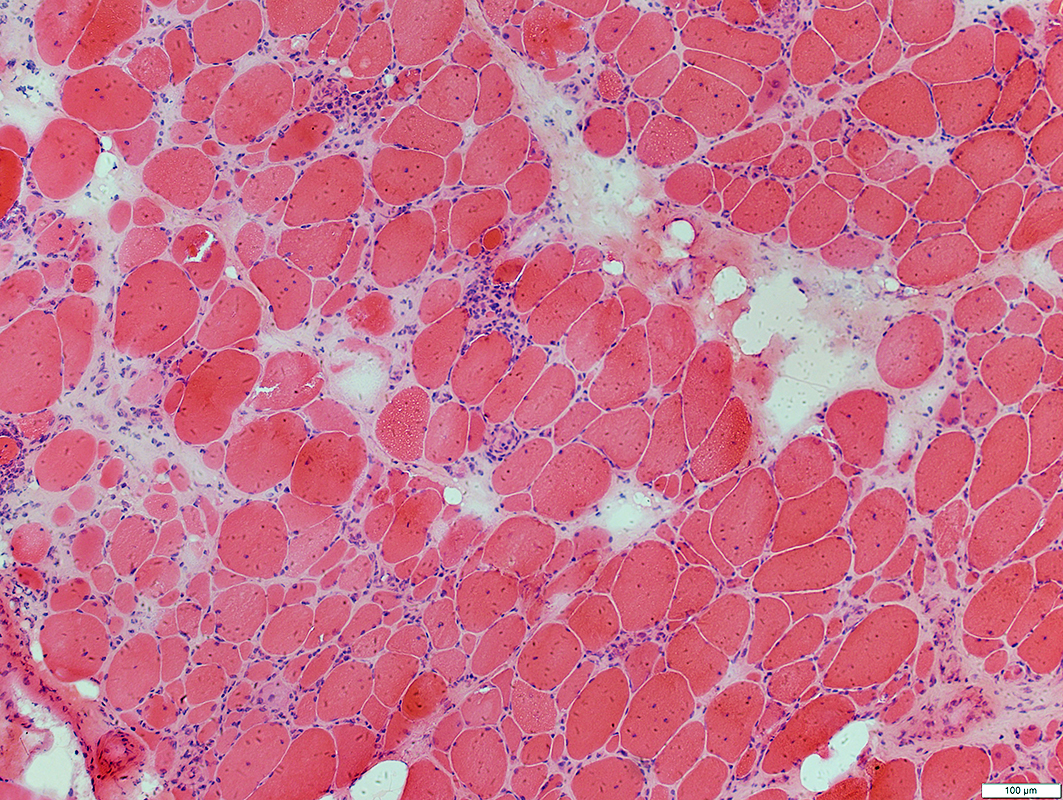
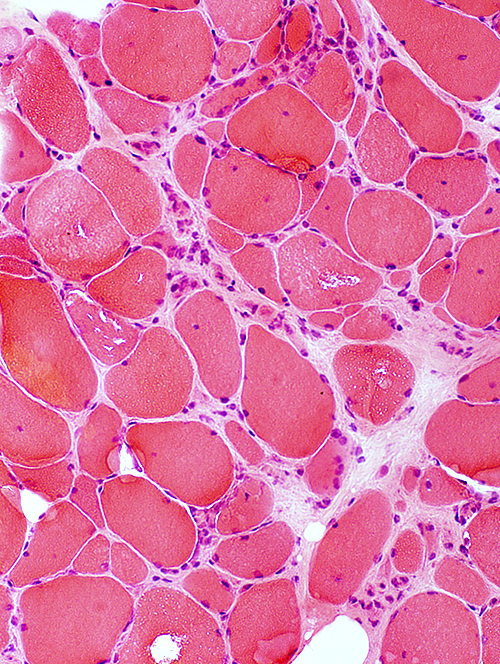
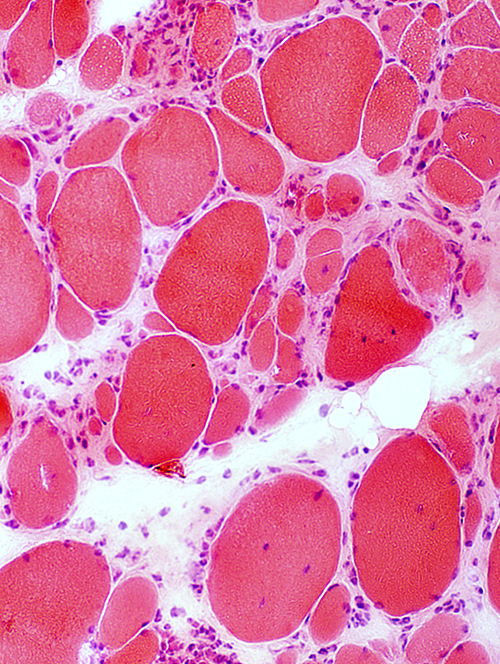
IBM: Myopathic features
 H & E stain |
 H & E stain |
 H & E stain |
Myopathy: Chronic; Ongoing
| |
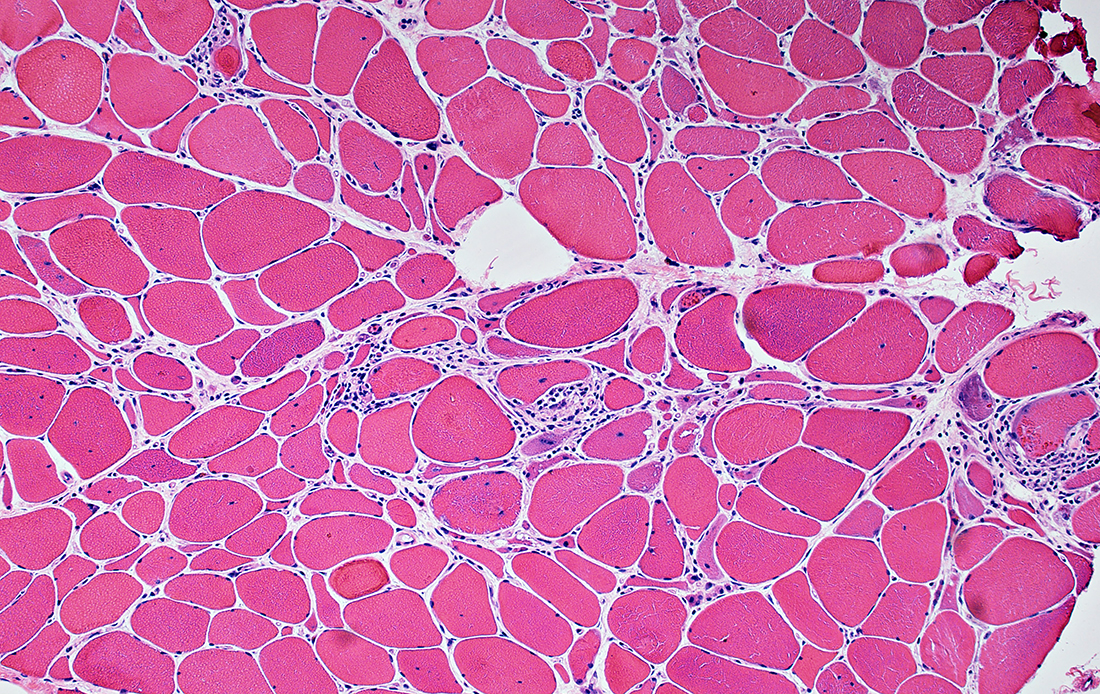 Fiber size: Varied Focal invasion by cells Vacuoles |
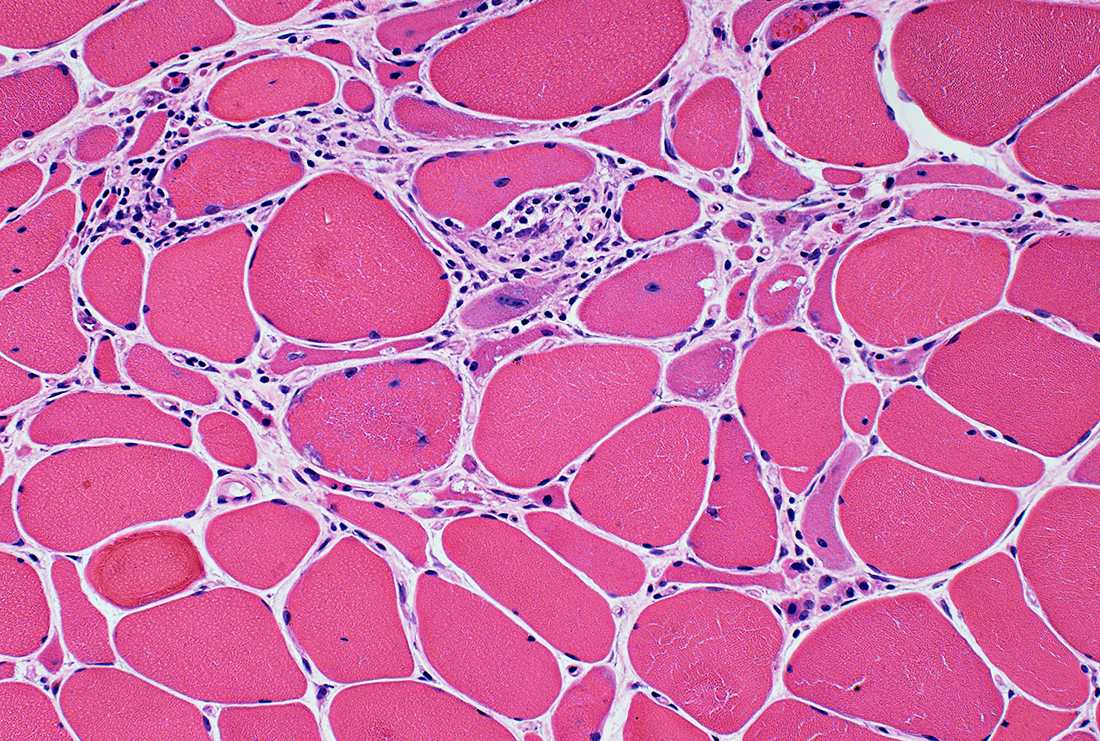
|
|
2C fibers (Intermediate-stained): Common 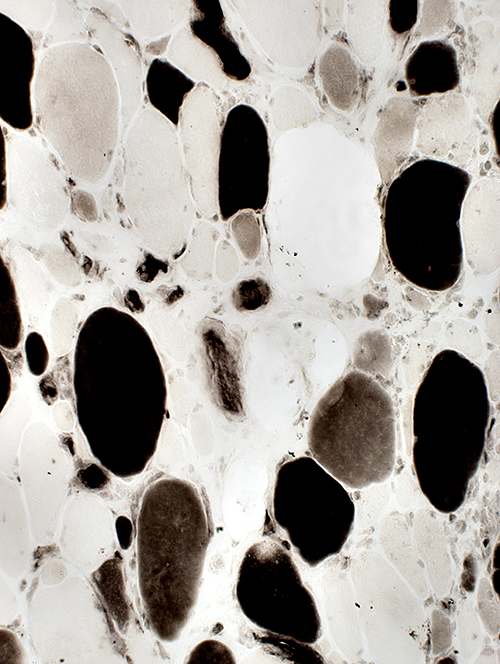 ATPase pH 4.3 stain |
Alkaline phosphatase stain: Often normal 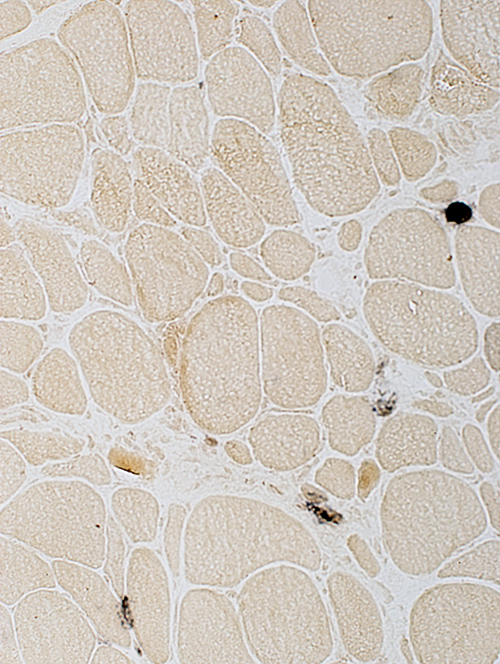 Alkaline phosphatase stain |
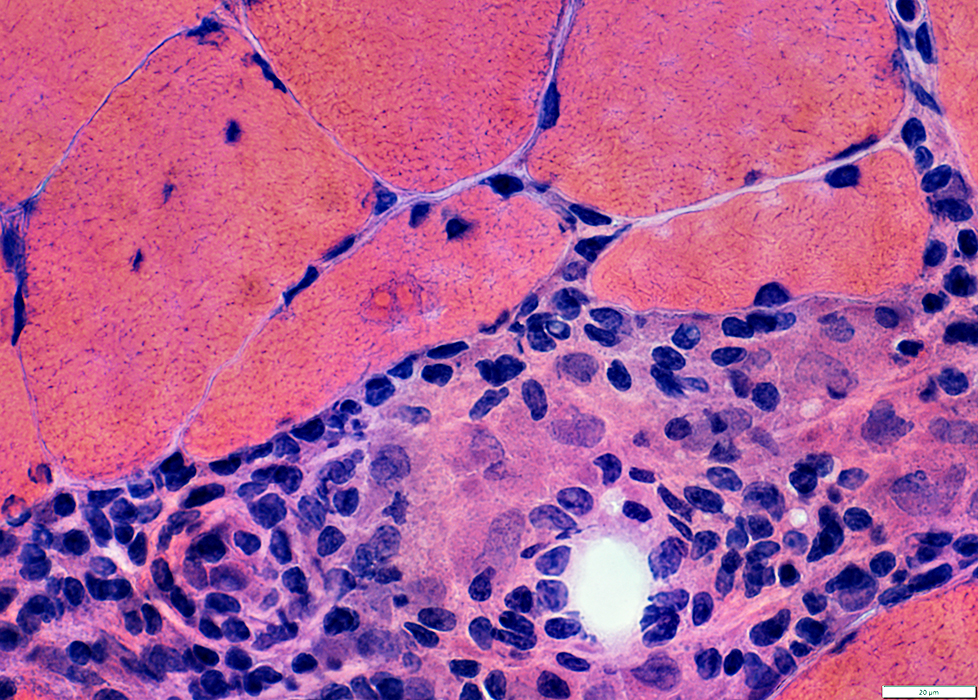
IBM: Inflammation
Focal invasion of Muscle fibers
Endomysial
Perimysial
Endomysial inflammation
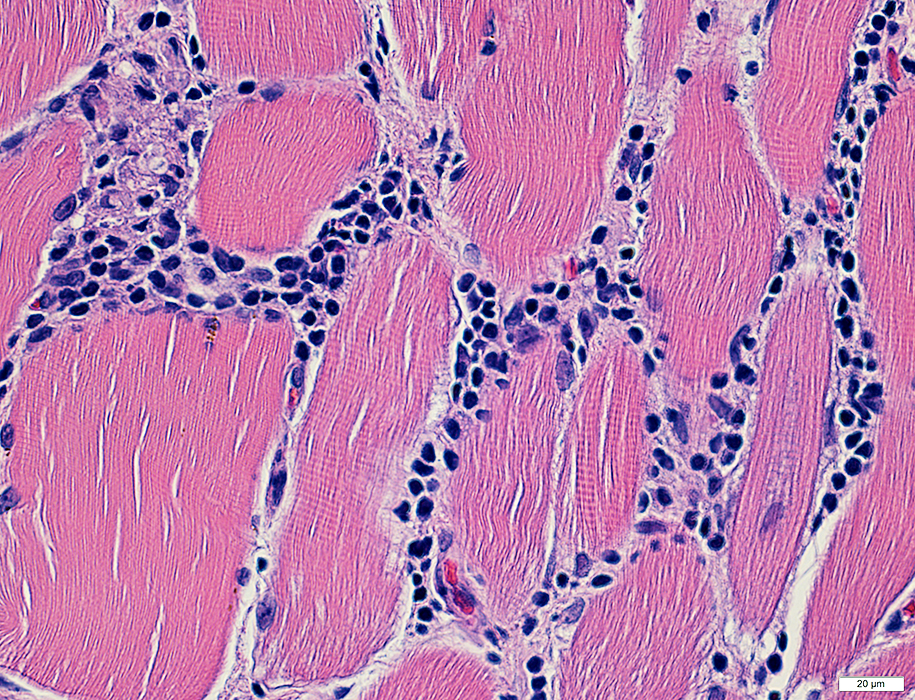 H&E stain |
Many cells are lymphocytic
Minority of cells are histiocytic (Acid phosphatase +)
Some immune cells are focally invading muscle fibers (Arrow)
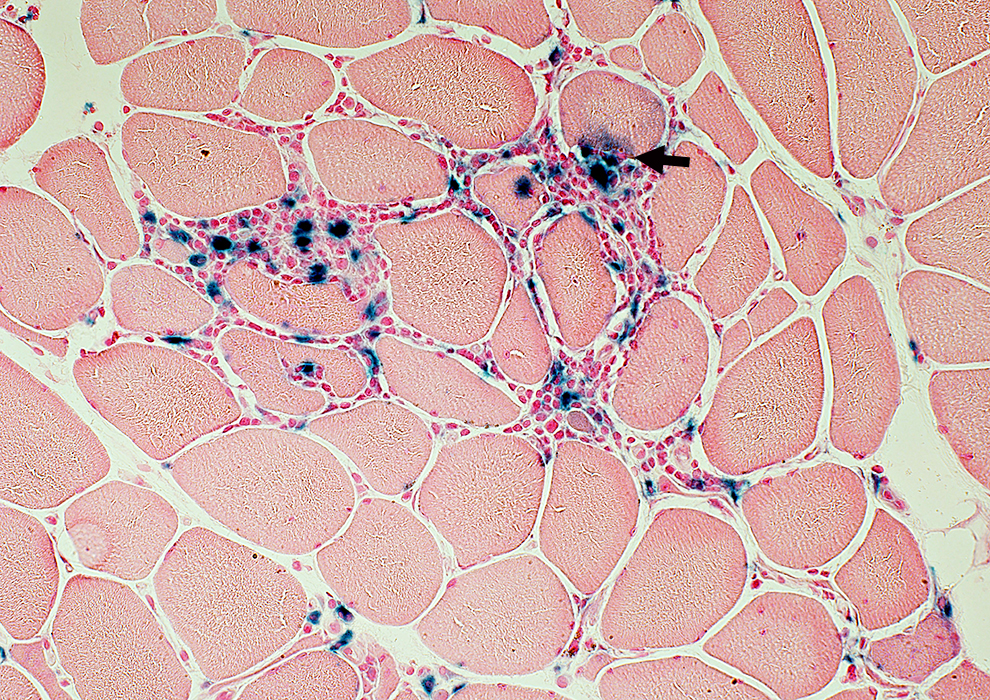 Acid phosphatase (Blue) stain |
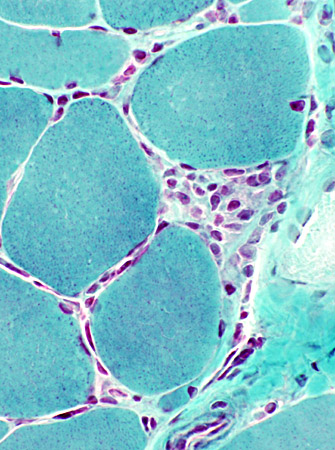
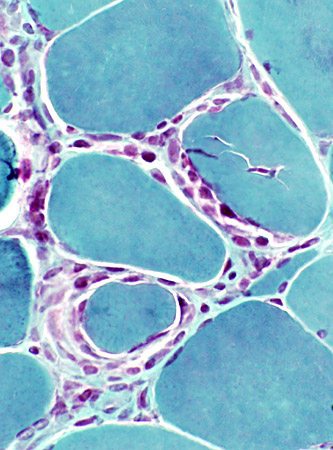 Gomori trichrome |
- Linear distribution of cells between muscle fibers (Above, Left; Below)
- Larger collections of cells around muscle fibers (Above, Right)
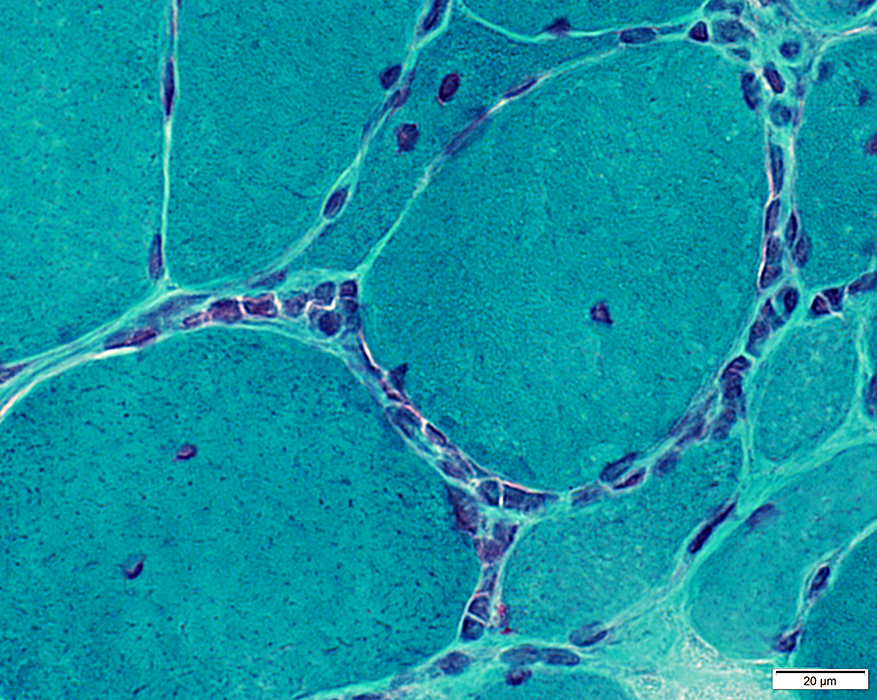 Gomori trichrome |
Inflammatory cells in endomysium
Cells are Focally invading a muscle fiber
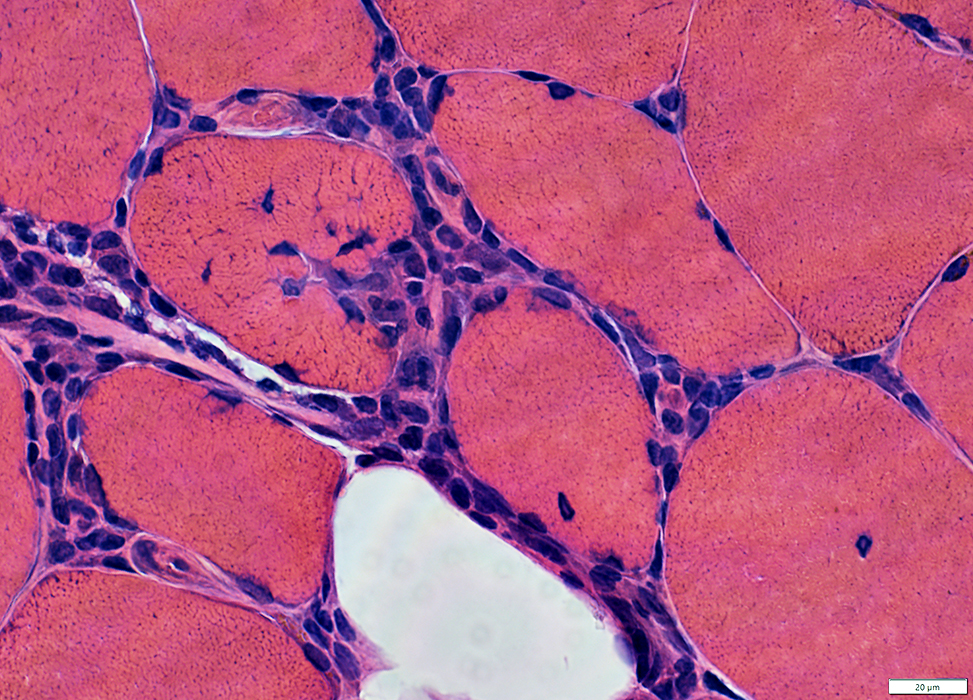 Congo red |
|
Inflammation: Endomysial & Perimysial Lymphocytes & Histiocytes in Endomysium Focal invasion of some muscle fibers by cells (Arrow) 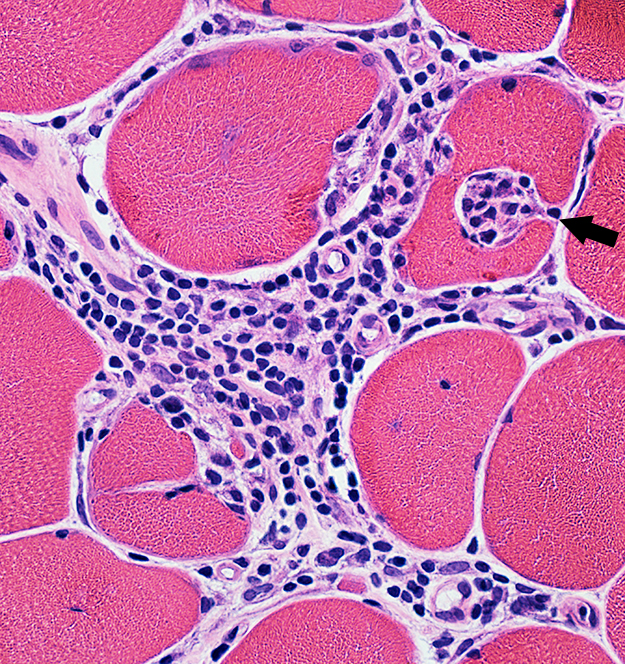 H & E stain |
Inflammation: Endomysial Focal invasion of some muscle fibers (Dark arrow) Partially fused (Split) muscle fiber (Light arrow) 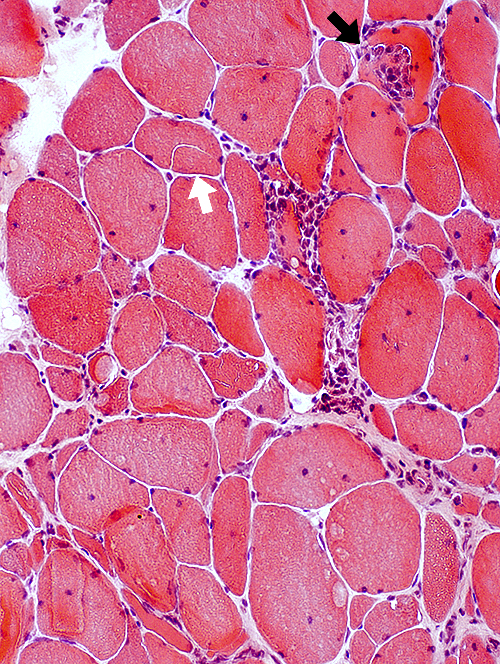 H & E stain |
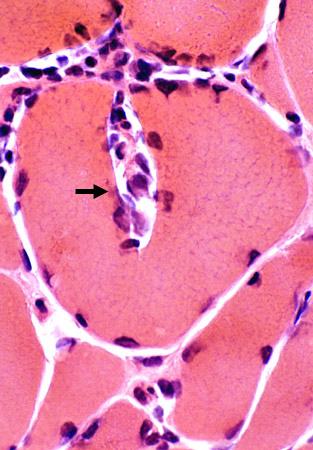 H & E stain |
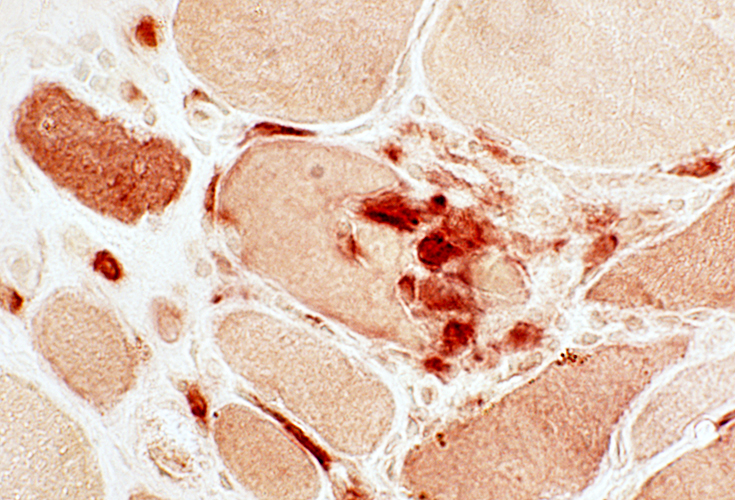 Esterase stain |
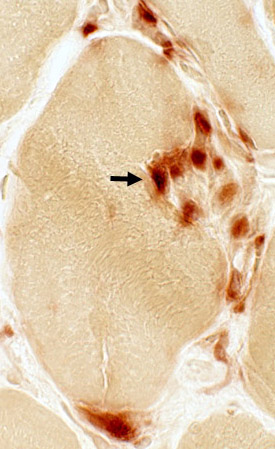 Acid phosphatase stain |
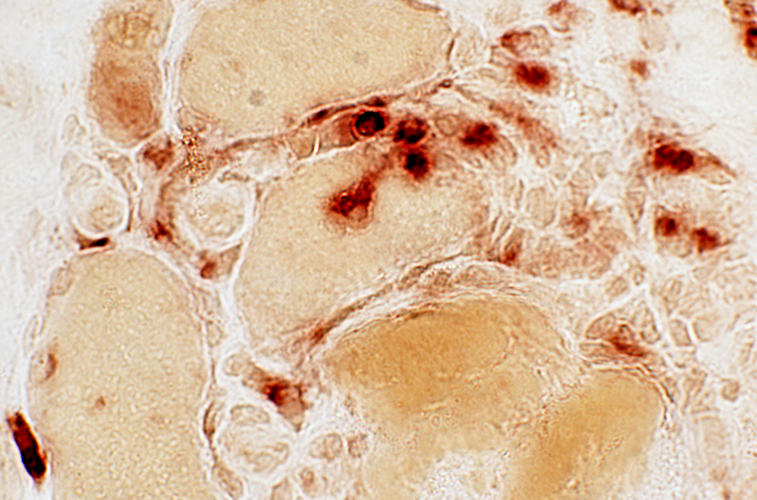 Acid phosphatase stain |
Focal invasion of muscle fibers
|
|
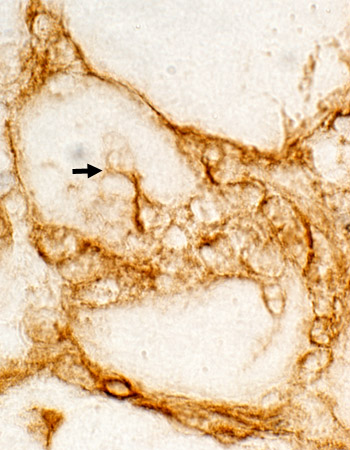 CD4 stain |
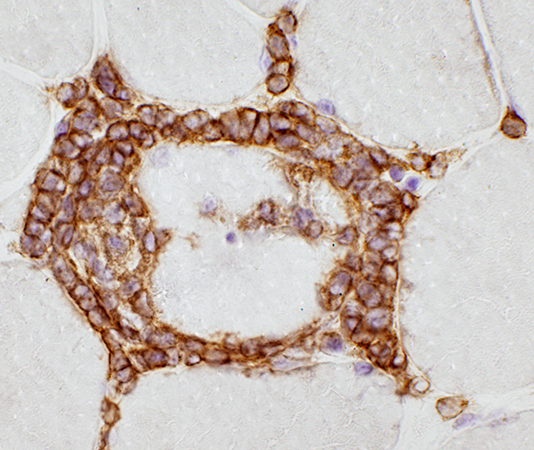 CD3 stain |
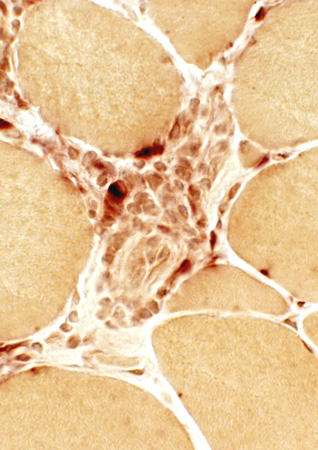 Acid phosphatase |
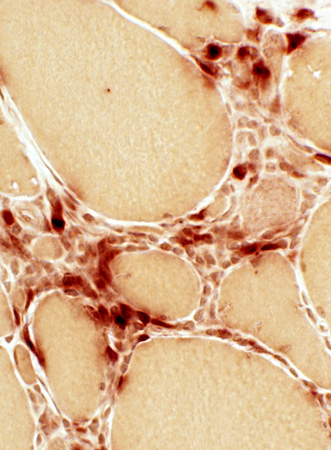 Acid phosphatase |
|
|
Muscle fibers replaced by immune cells
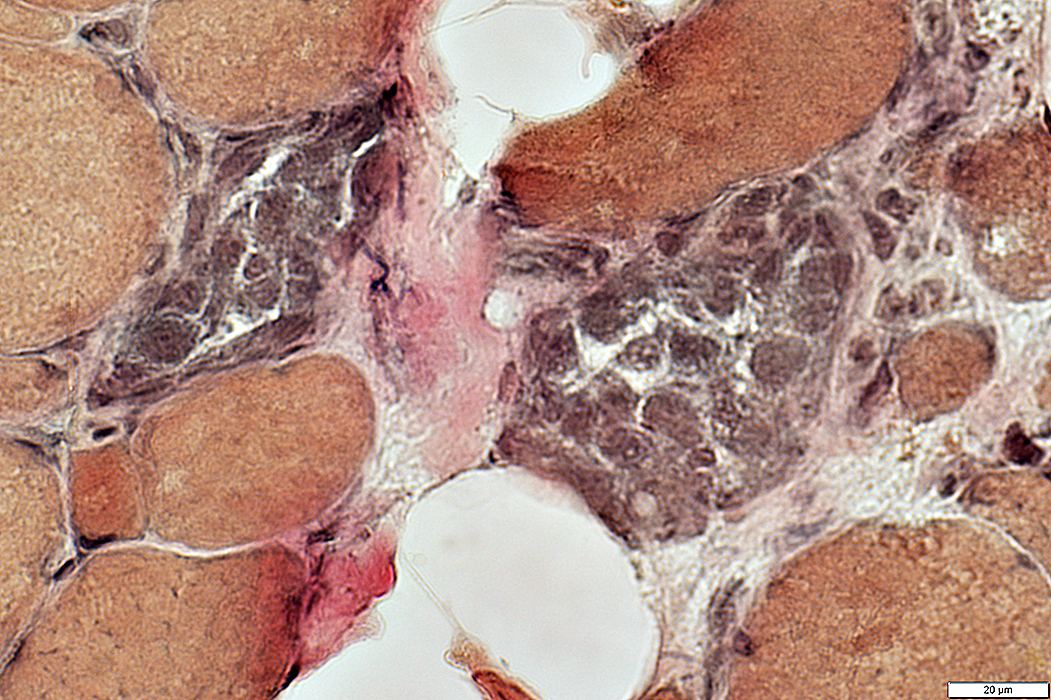 VvG stain |
IM-VAMP (IBM): Lymphocyte Types
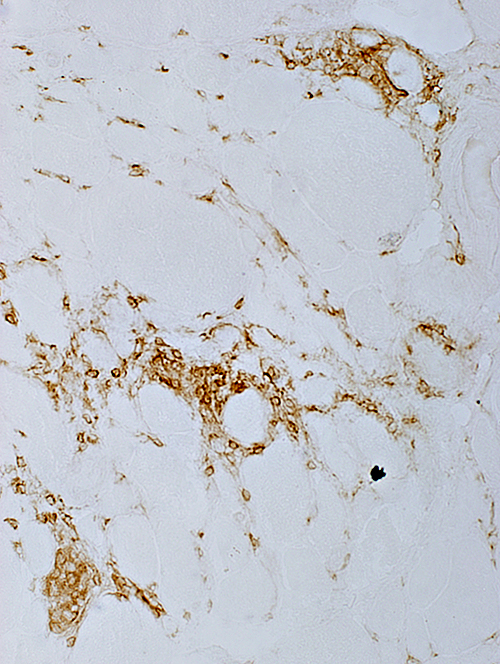 CD4 CD4 cells: Present in foci & Scattered in endomysium |
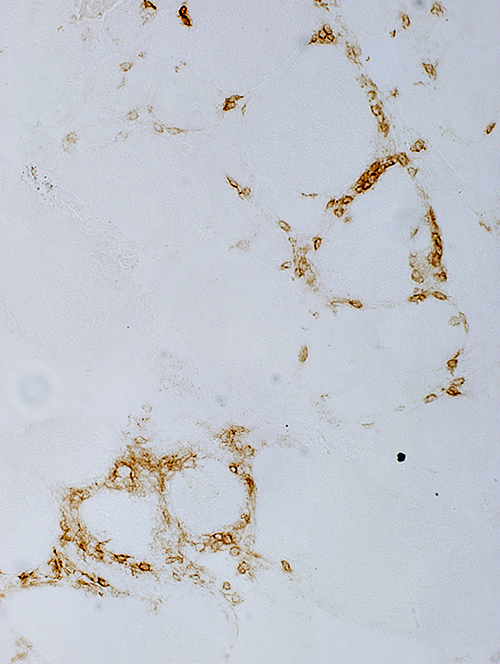 CD8 CD8 cells: More in foci than in other endomysial regions |
IM-VAMP: Cell types
Lymphocytes: CD4 & CD8 cells
Present in endomysial lymphocyte clusters
CD8 cells
Location
Endomysial cell foci
Focal invasion of muscle fibers
Terminally differentiated (TEMRA) phenotype
Loss: CD28
Upregulation: Killer cell lectin-like receptor G1 (KLRG1) & CD57
Cytotoxic potential: Perforin; Granzyme B; KLRG1
Limited proliferative capacity
May produce IFN-γ
Large granular lymphocytes (LGLs; CD3+, CD8+, CD57+, CD244+, CD28-, Kv1.3+)
Clinical association: Large granular lymphocytic (T-LGL) leukemia
CD4 cells
Scattered: In endomysium between muscle fibers
CD4+CD28(null) T cells: Endomysial cell foci; Proinflammatory & Cytotoxic
T cell receptor (TCR) Vβ repertoire
Restricted (Clonal expansions)
Similar to changes seen in blood
Histiocytic cells
Scatttered: In endomysium
Clusters: Locations
Endomysial regions of focal invasion of muscle fibers by cells
Surround muscle fibers
Types 6
M1 macrophages: Near venous endotheial cells, Reactive & Damaged fibers, NMJs
M2 macrophages: Near LAMP3 Dendritic cells, Plasma cells, Type 2 fibers, Adipocytes
Plasma cells: Few scattered in endomysial cell foci
B-cells: NOT present in cell foci
Fibro-Adipogenic Progenitor (FAP) cells 6
Nerve-associated Fibroblasts
Molecules: NLGN1, NEGR1
Cell associations: Normal & Damaged muscle fibers, M2 macrophages
Damaged Muscle Fibers
Molecules: GADD45A, NORAD, AChE
Features: Atrophy, Cell/genomic stress, Associated T-cells
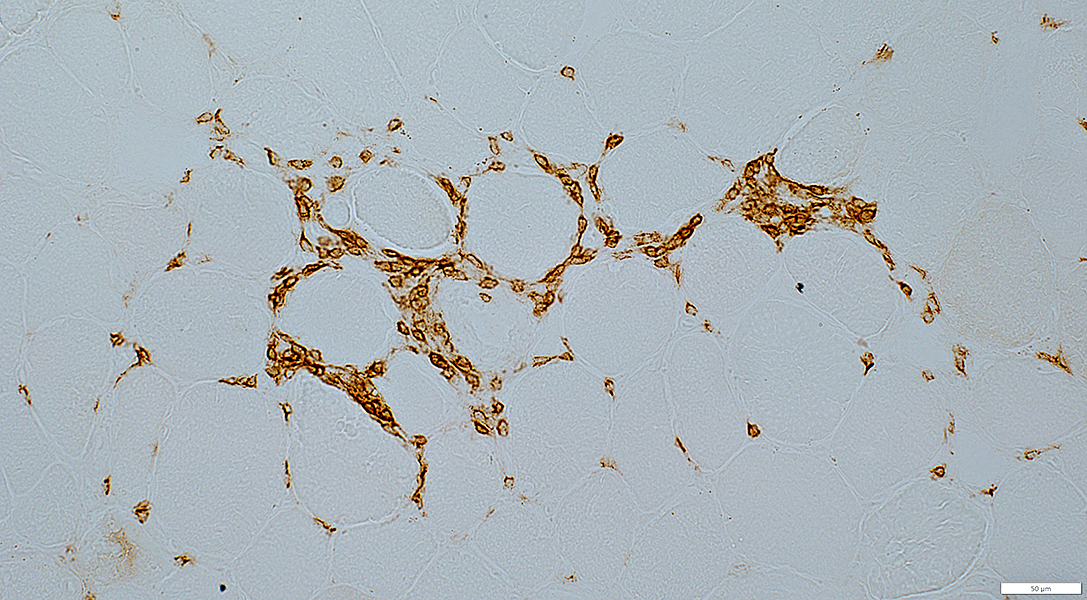 CD8 stain |
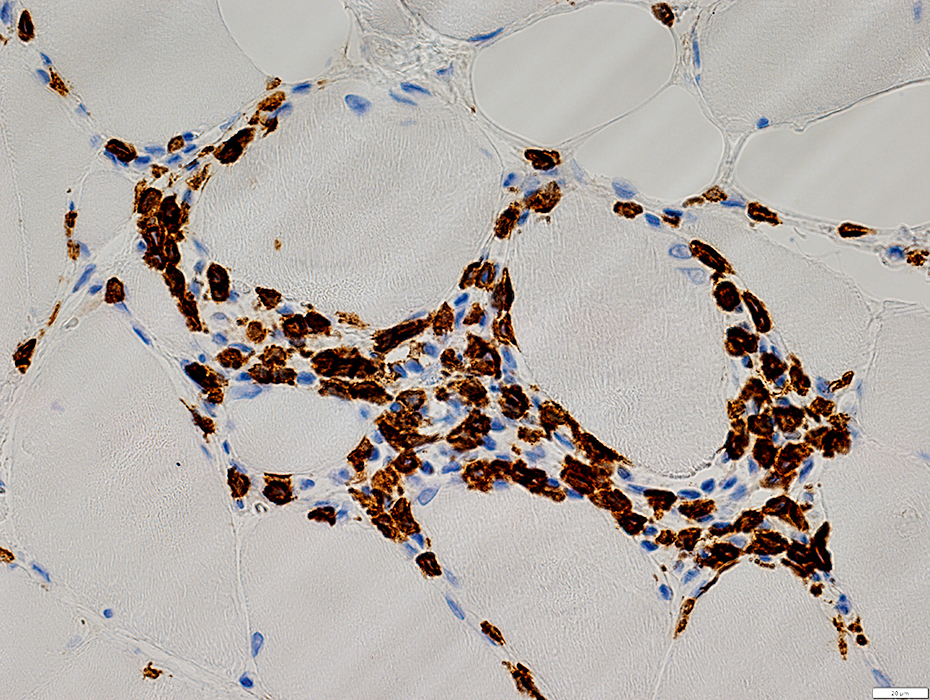 CD8 stain |
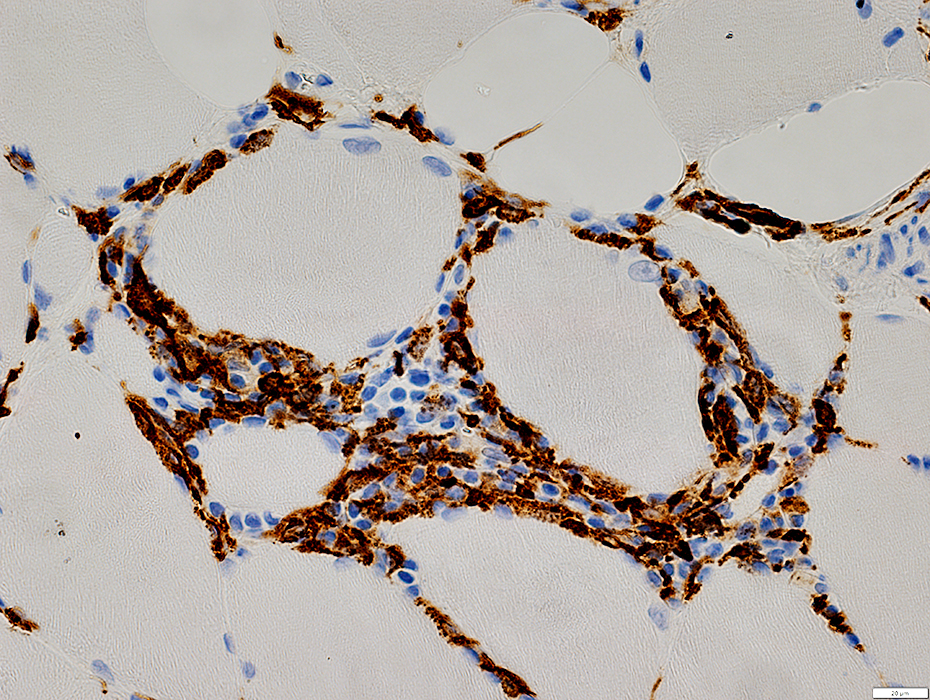 CD163 stain |
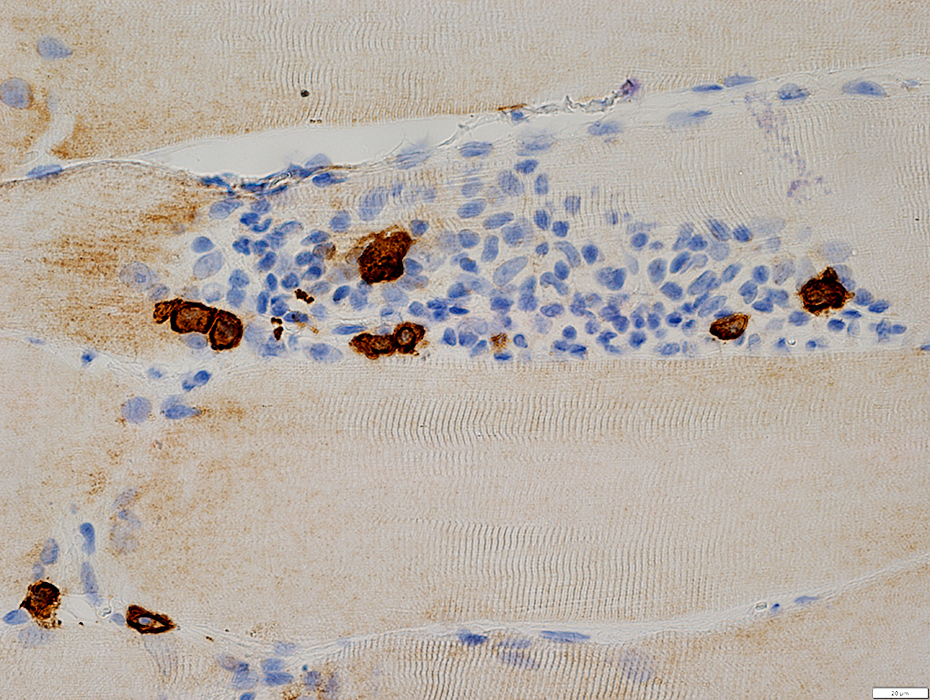 CD138 stain |
IM-VAMP (IBM): Perimysial inflammation
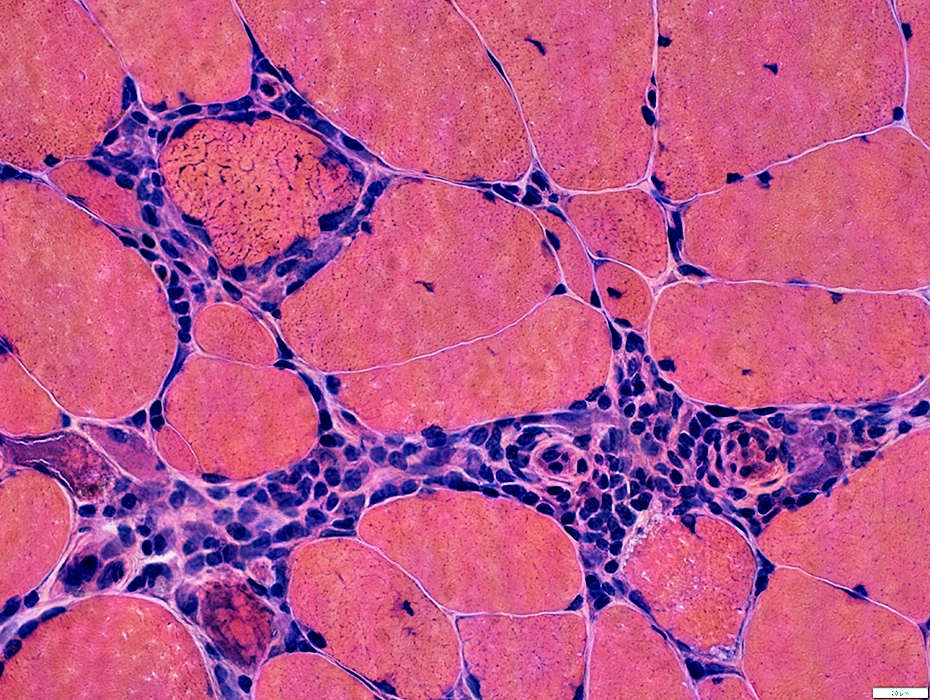 H&E stain |
Cells: Many lymphocytes
Locations
Endomysial (Above): Surrounds a muscle fiber
Perimysial (Below): Contains small vessels
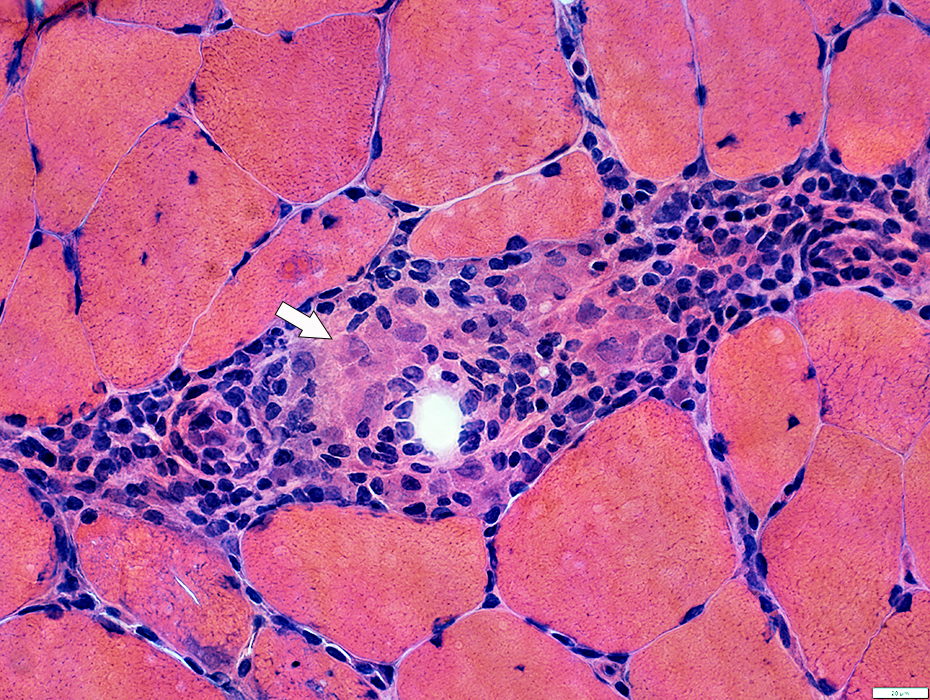 H&E stain |
Cells: Granuloma-like histiocytes (Arrow, Above; Esterase+, Below) Surrounded by lymphocytes
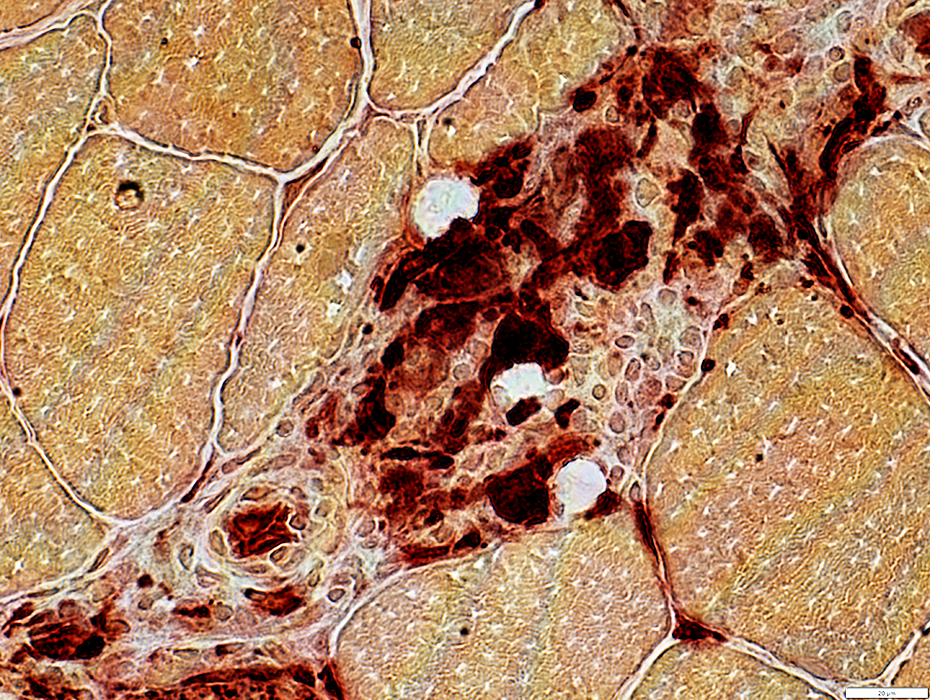 Esterase stain |
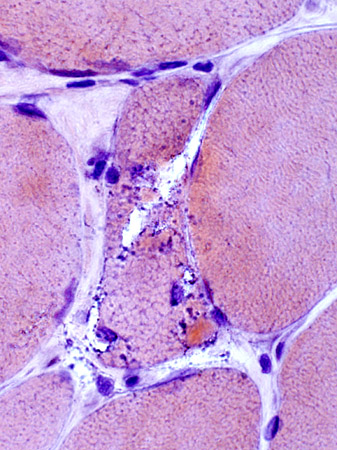
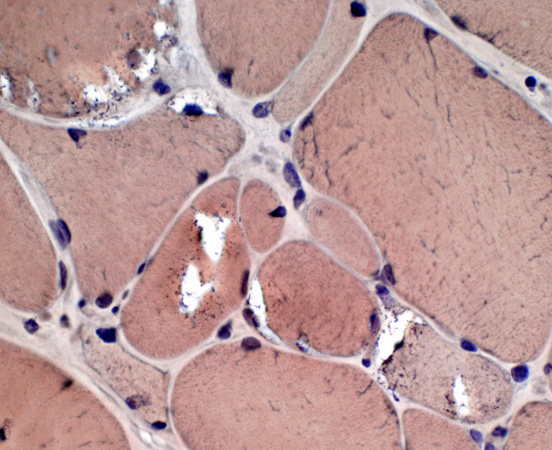
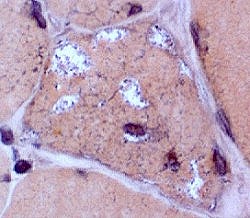
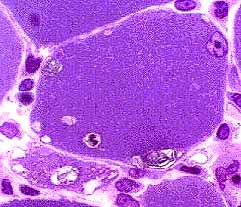
IBM: Vacuoles (or Inclusions)
Vacuoles- Muscle fibers with several rimmed vacuoles (arrows)
- Vacuoles contain
- Congo red: Blue amorphous, granular material
- Gomori trichrome: Red-stained material in muscle fibers & around vacuoles
- VvG: Gray-stained material
- Nuclei: Some are near vacuoles or enlarged
- Amyloid in some vacuoles
- Visualized with polarized light
- Small foci of red stained amyloid (arrows) are in, or around, vacuoles
- Some muscle fibers also contain
- Cytoplasmic bodies or inclusions
- Differential diagnosis: Vacuoles
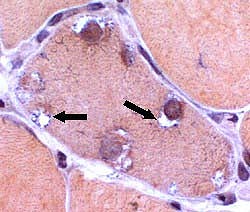 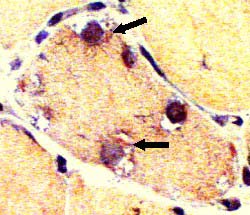 Congo red stain |
 Congo red stain |
 Congo red stain |
Shape irregular
Granular basophilic debris
Often within, and surrounding, vacuoles
May have amyloid-like, Congo red birefringence
 Congo red stain |
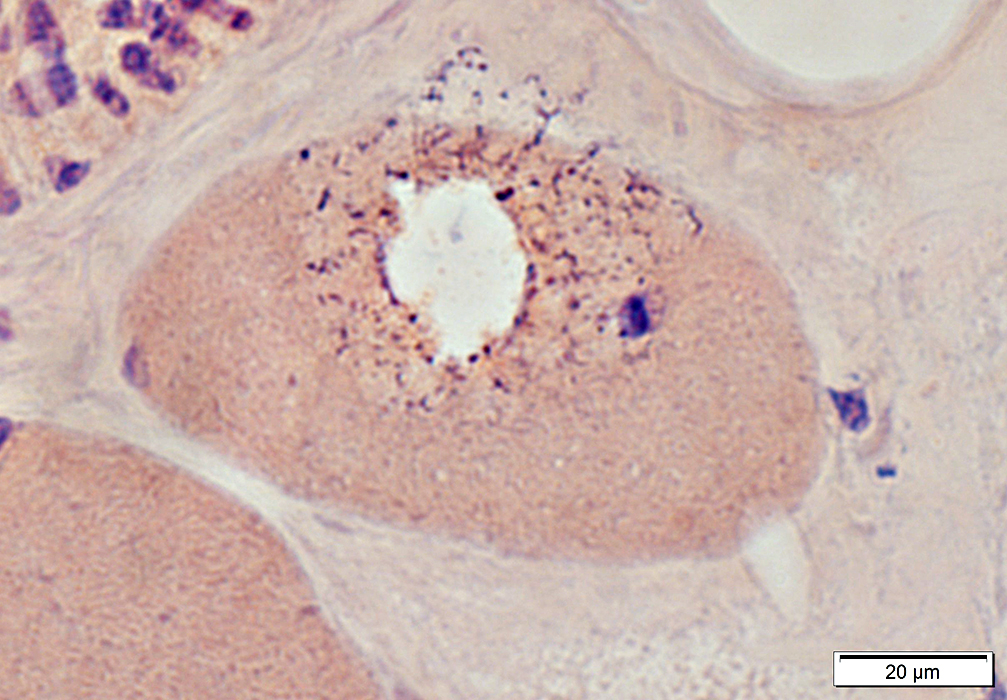
In regions surrounding large vacuole
|
Vacuoles
|
 Gomori trichrome stain |
  |
|
IBM: Vacuoles One, or several, in a muscle fiber Shapes: Irregular Some contain red-staining material 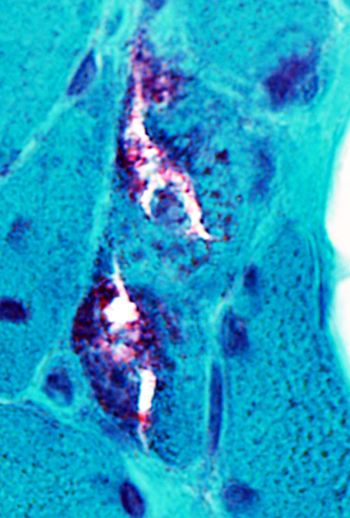
|
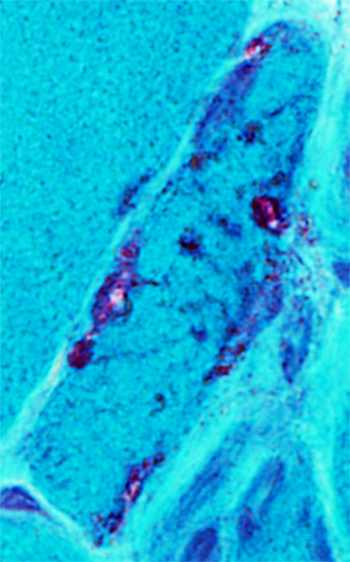
|
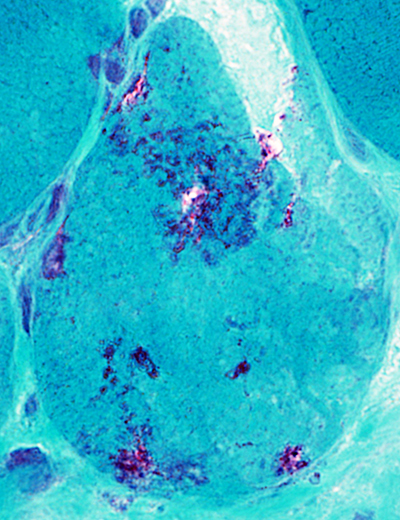 Gomori trichrome stain |
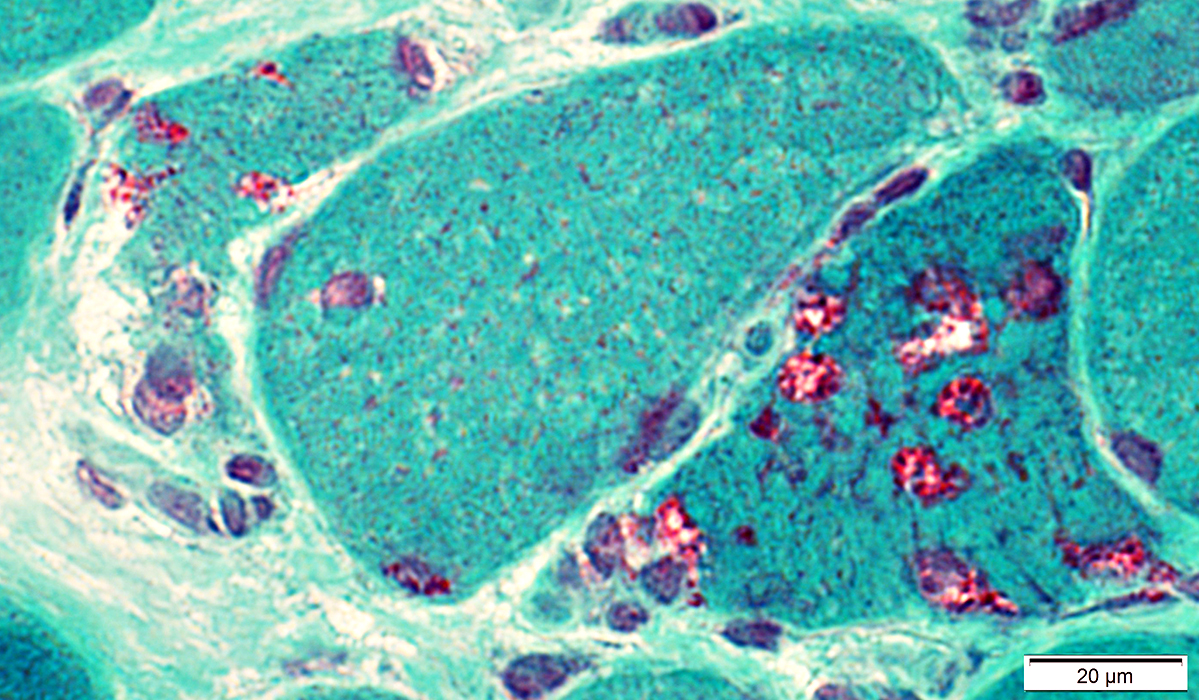 Gomori trichrome stain |
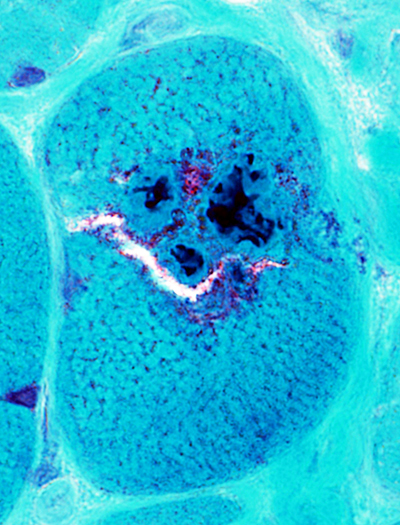
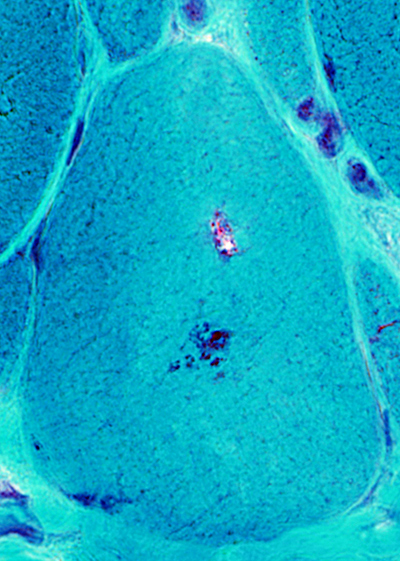
IBM aggregates: Cytoplasmic Bodies
 Gomori trichrome stain |
 Gomori trichrome stain |
|
Cytoplasmic bodies (Arrow) may occur in: Muscle fibers with, or without, vacuoles
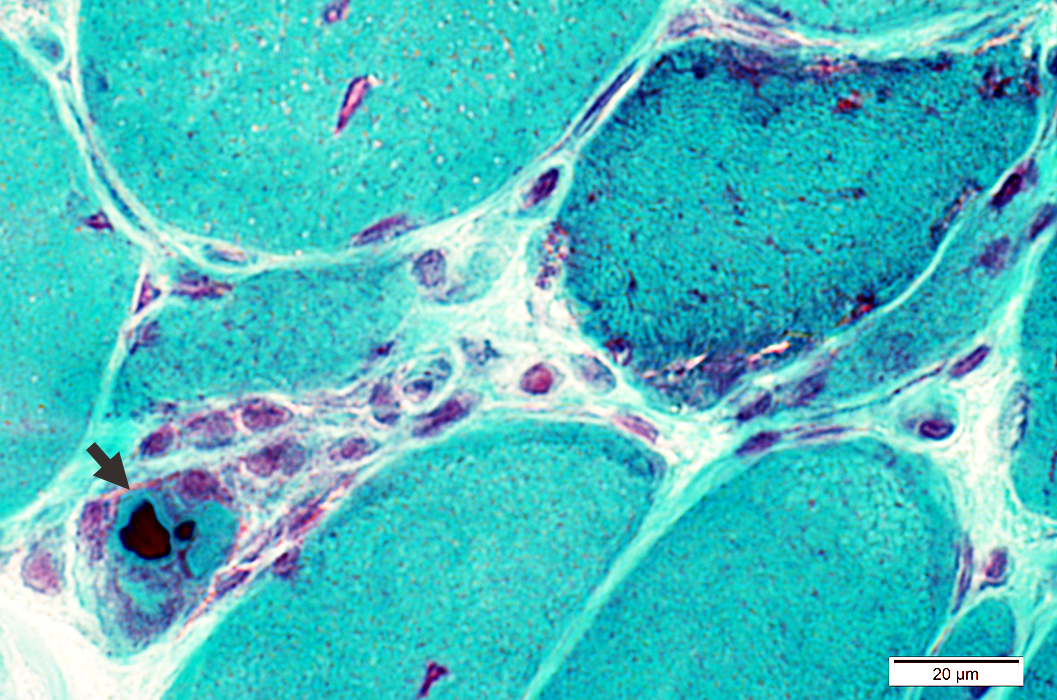 Gomori trichrome stain |
Atypical vacuoles
Contain irregular green-stained material
Larger than usuallyh found in sIBM
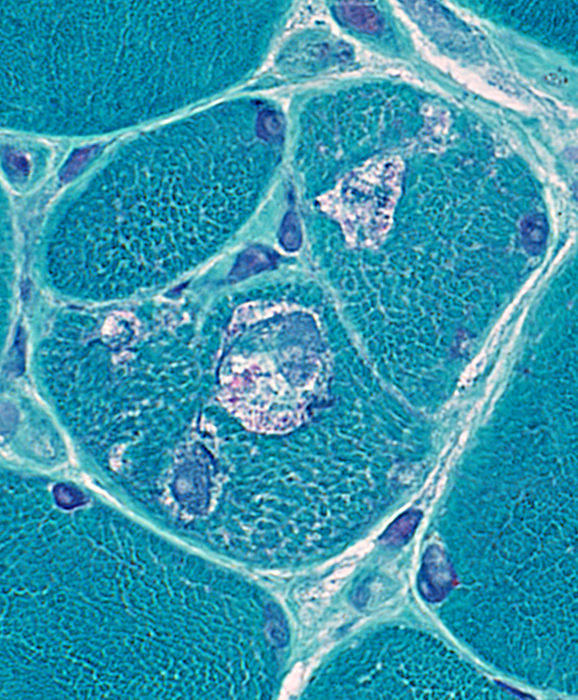 Gomori trichrome stain |
 Gomori trichrome stain |
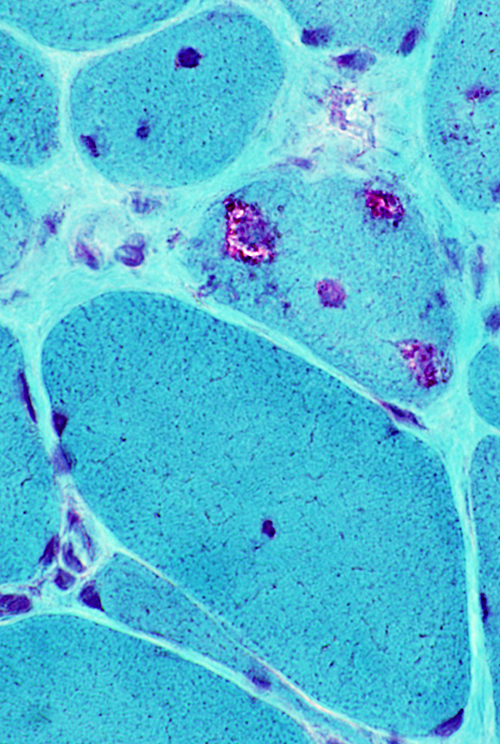
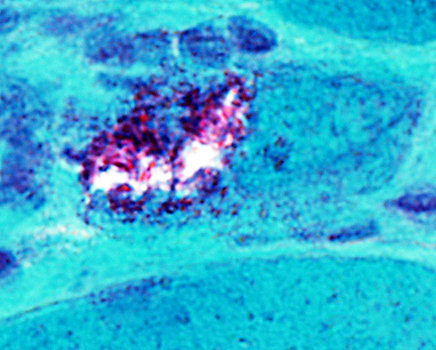
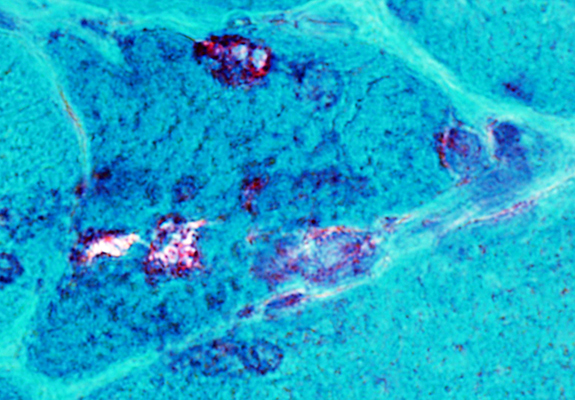
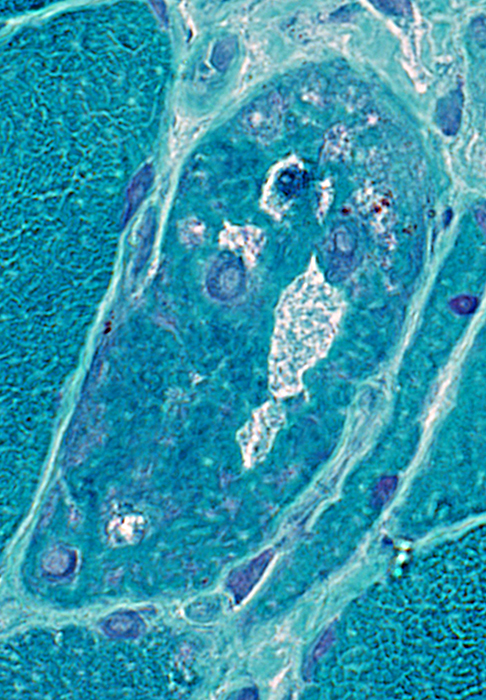
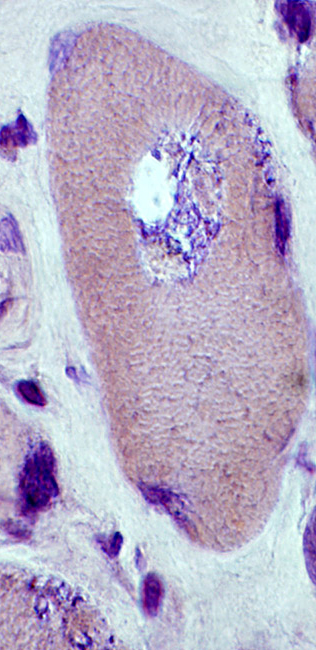
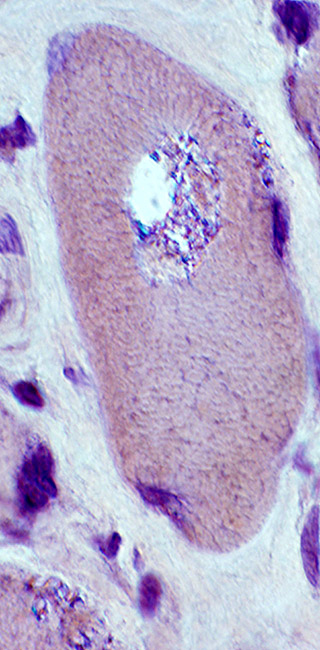
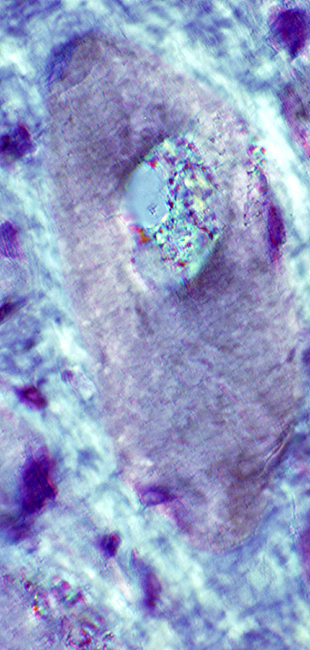
IM-VAMP: Amyloid
|
|
|
- Muscle fiber with large vacuole (Left)
- Birefringence: Red-Green material in, and near, vacuole (Middle & Right)
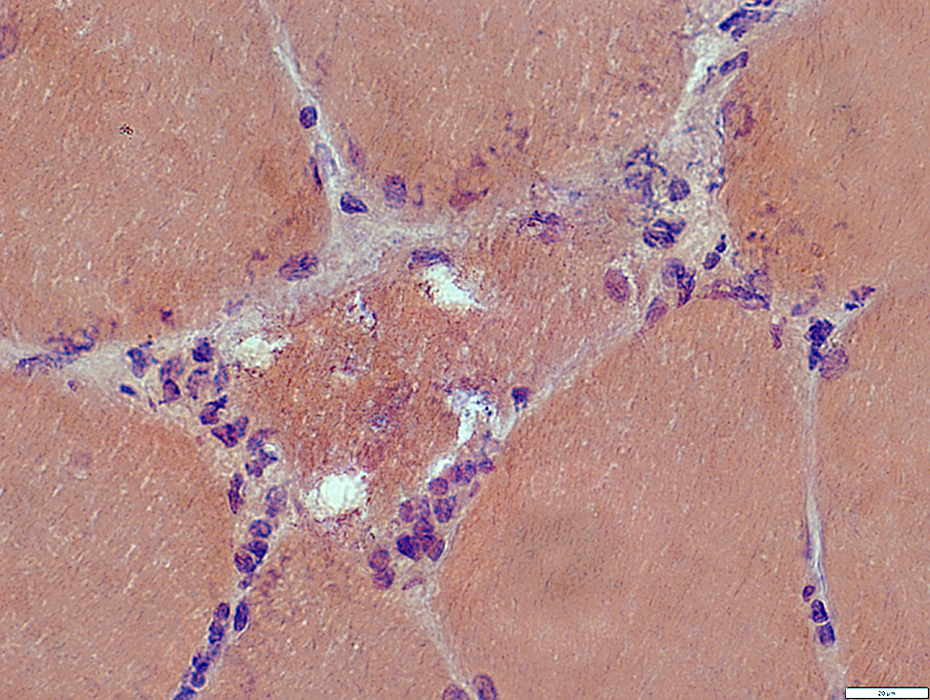 Congo red stain |
Granular basophilic debris near vacuoles may have red-green birefringence (Below)
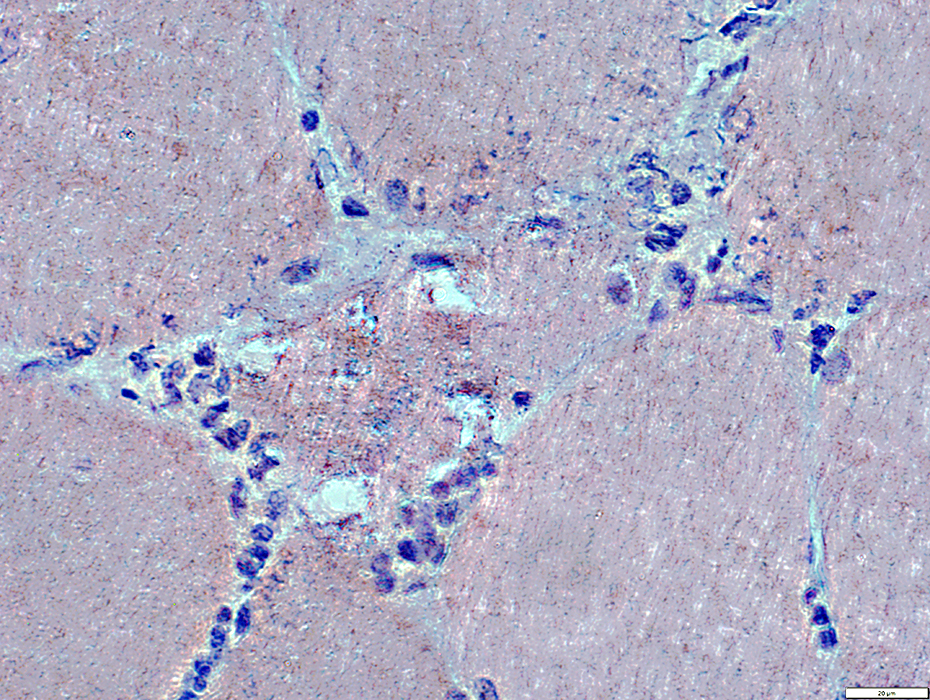 Congo red stain |
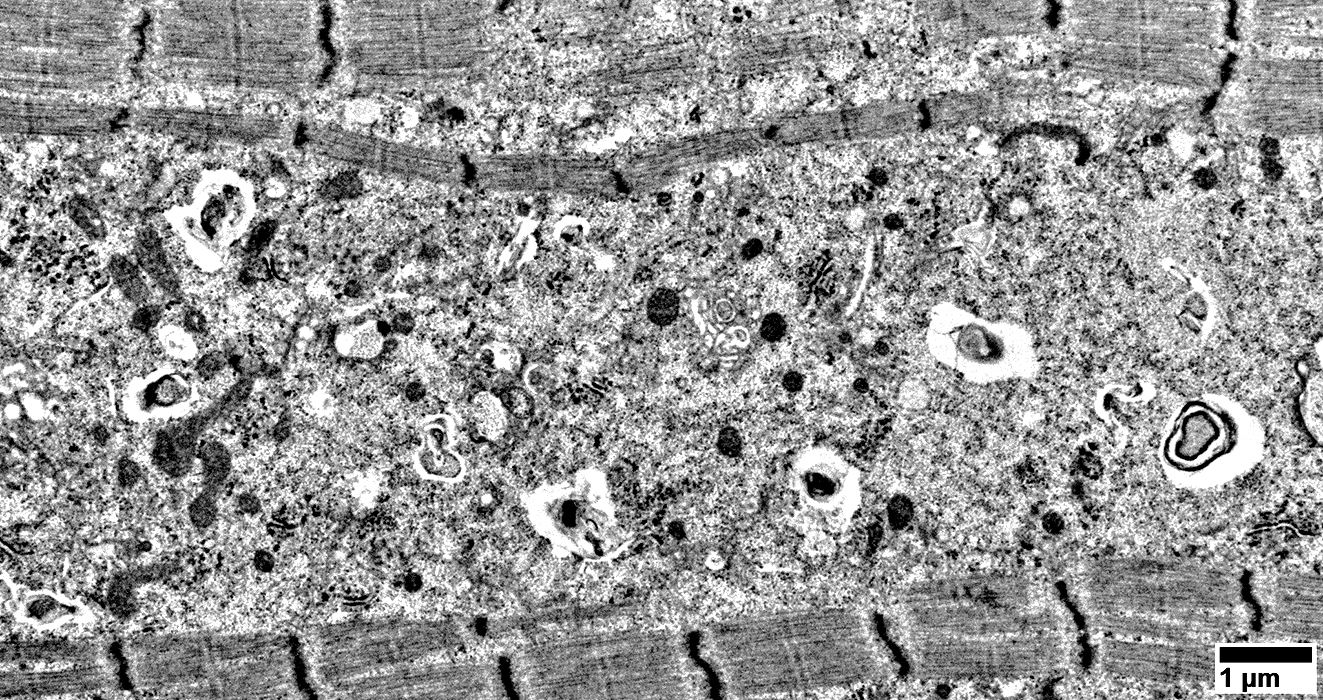 From: R. Schmidt |
Contents include: Filaments; Scattered mitochondria;
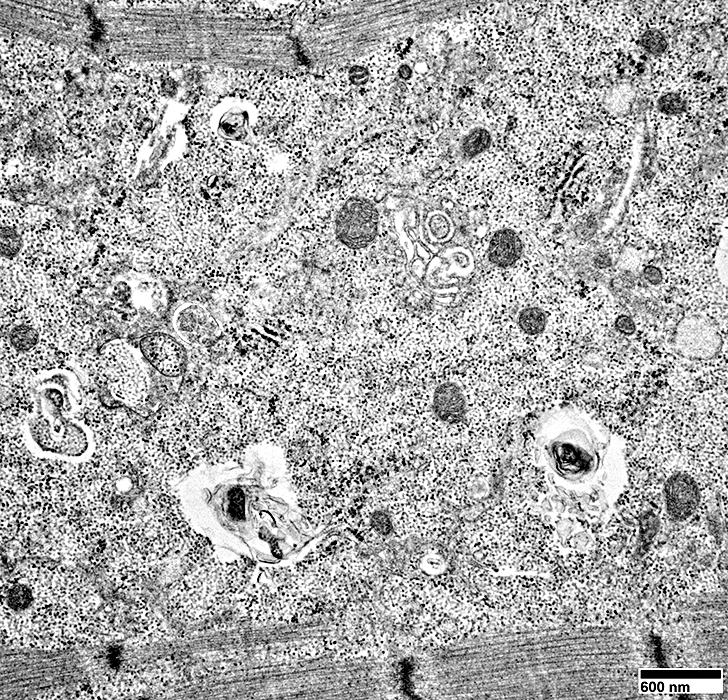
From: R. Schmidt |
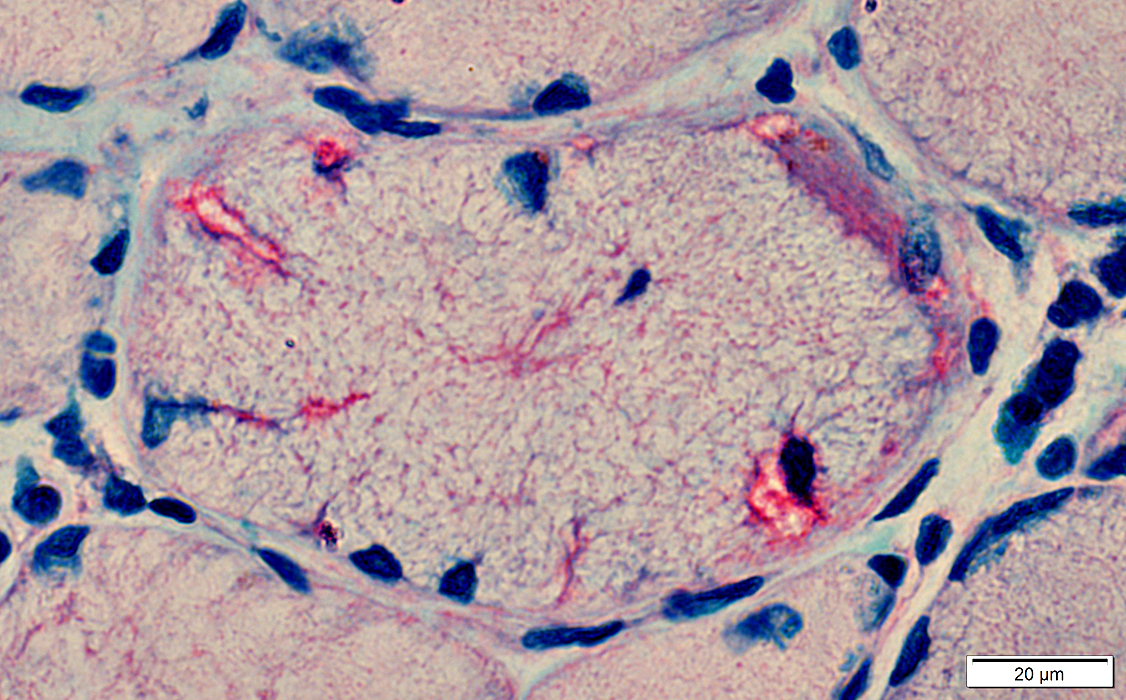 Alcian blue/Nuclear fast red |
IBM: Aggregates
Aggregates: General features in IBM-like disorders- Location
- Most easily seen in the cytoplasm
- Minority occur neighboring nuclei or vacuoles
- Histochemistry: Stain with AMPDA, H&E & Gomori trichrome
- Contents
- Common components: SMI-31; TDP-43; LC3; αB-crystallin; p62
- Different components may occur alone or associated with other components
- None are very sensitive for the spectrum of IBM-like disorders
- LC3 aggregates
- Frequent in IBM/IM-VAMP
- Multiple small punctate aggregates
- LC3 aggregates occur in other muscle disorders but have different shapes & sizes
- SMI-31 aggregates
- May be the most specific for IBM
- Low sensitivity
- Emerin is not present in aggregates or vacuoles
- Cytoplasmic bodies
- Increased frequency in IBM/IM-VAMP
- May occur in fibers that also have vacuoles
Aggregates: Histochemistry
|
|
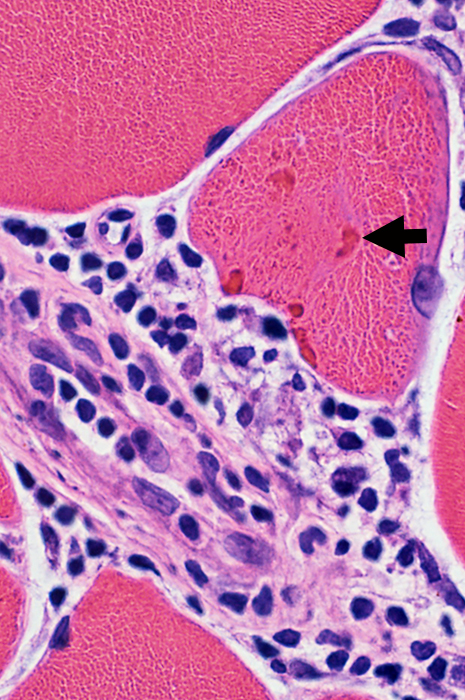
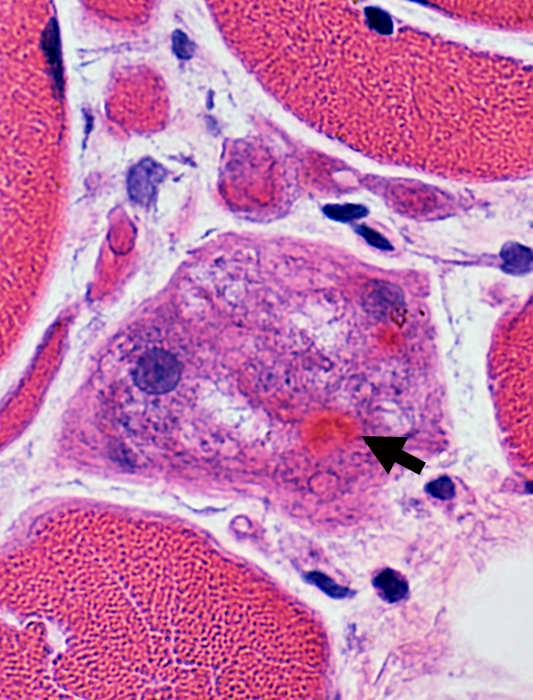
Cytoplasmic Aggregates Dark, eosinophilic hyaline appearance (Arrows) |
Cytoplasmic Aggregate Large Near irregular vacuoles |
|
 H&E stain |
 H&E stain |
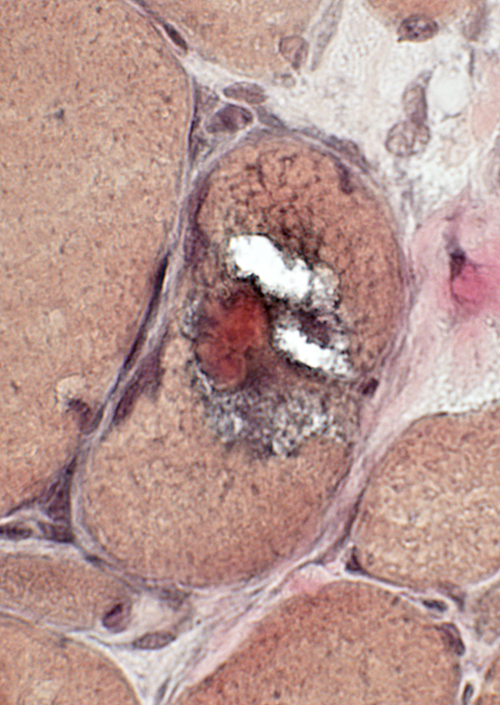 VvG stain |
Cytoplasmic bodies
Small, dark-stained structure
May be
Several in individual fibers
Present in fibers with or without vacuoles
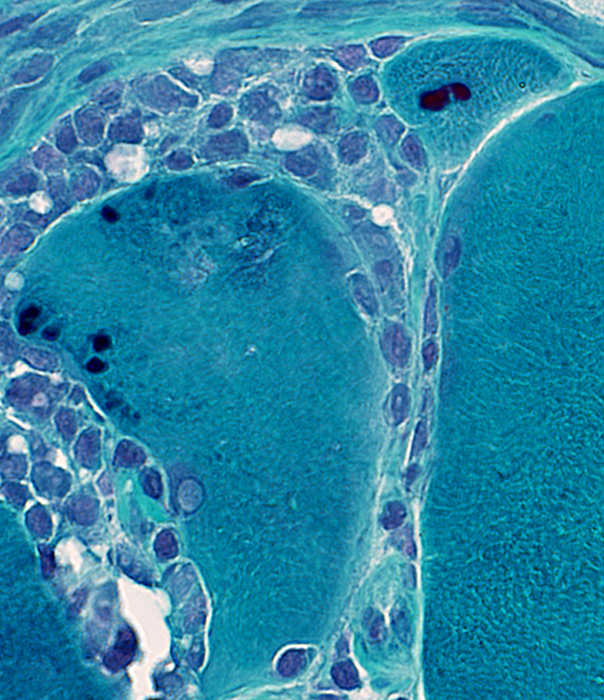 Gomori trichrome stain |
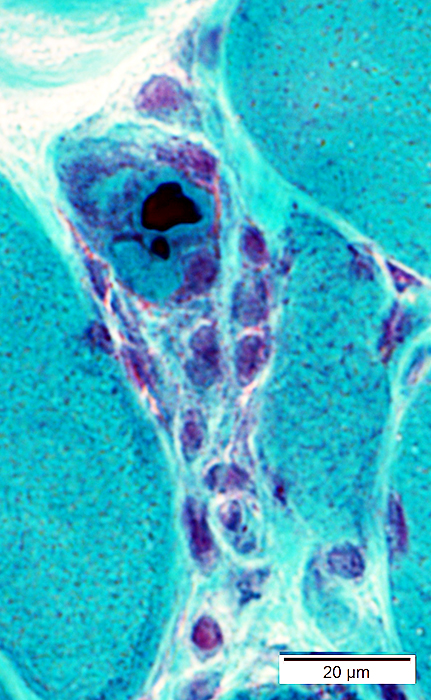 Gomori trichrome stain |
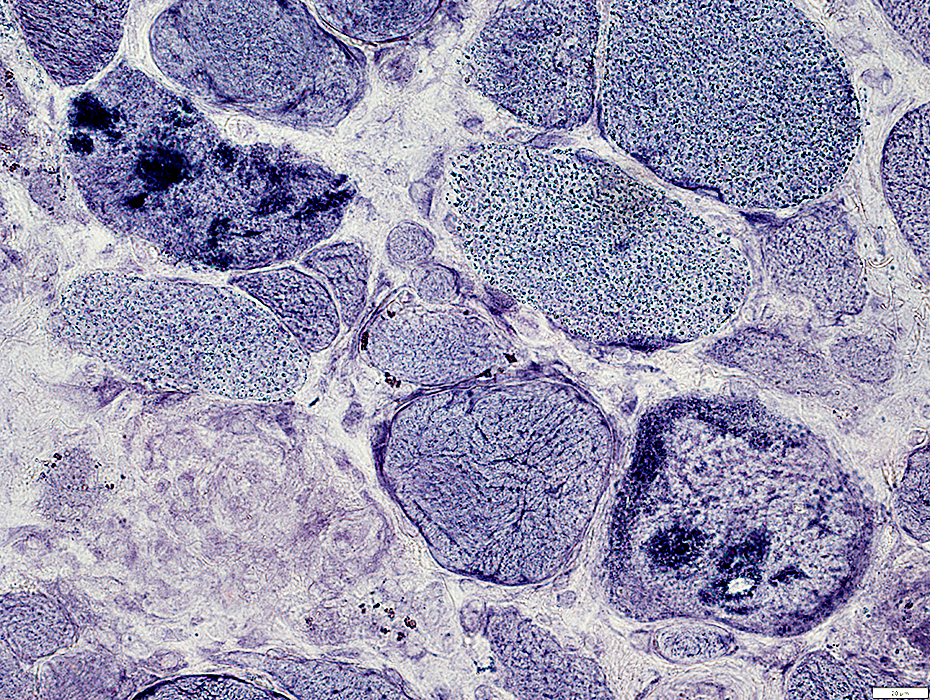 AMPDA stain |
Present in aggregated material in muscle fiber cytoplasm
Some, but not most, aggregated material surrounds vacuoles
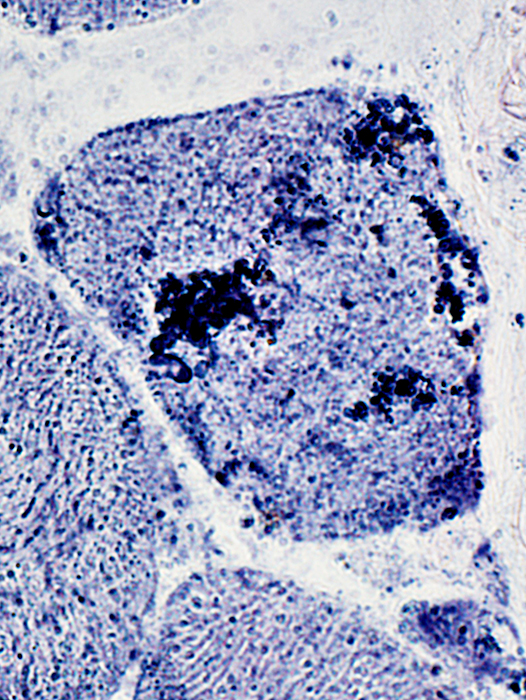 AMPDA stain |
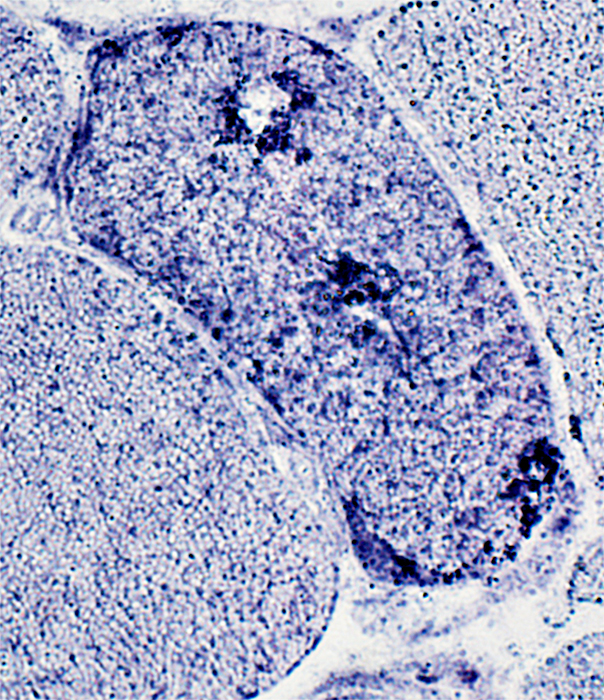 AMPDA stain |
SMI-31 positive aggregates in IBM
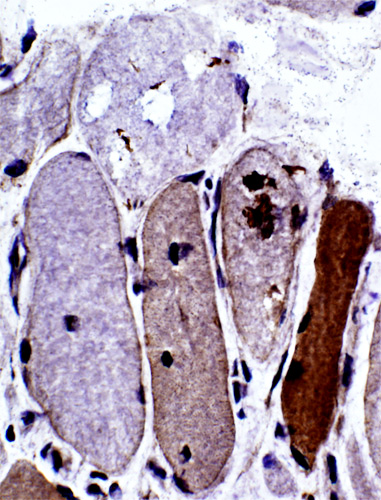 SMI-31 + Congo red stains |
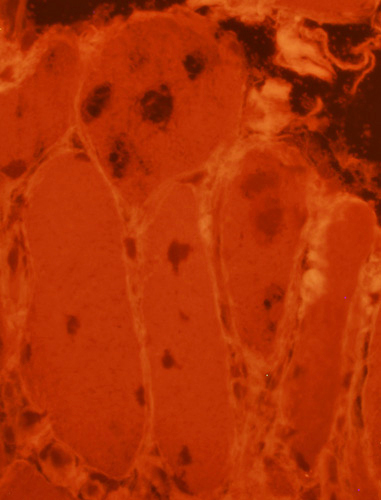 Congo Red + Fluorescence with Texas red filter |
|
|
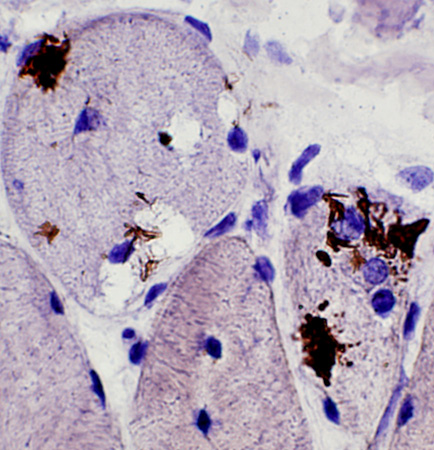 SMI-31 + Congo red stains |
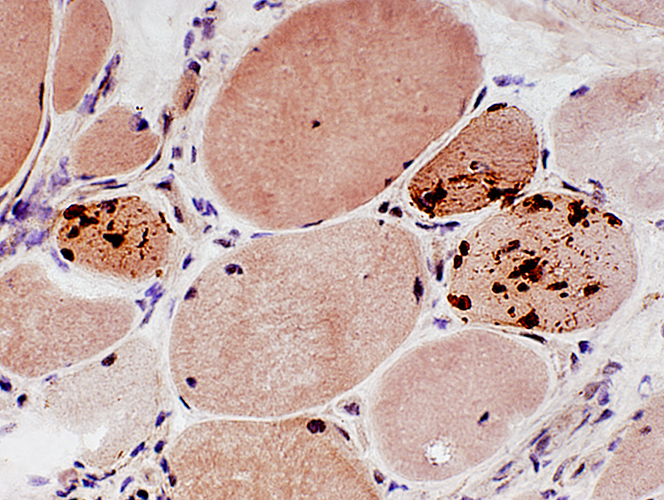
|
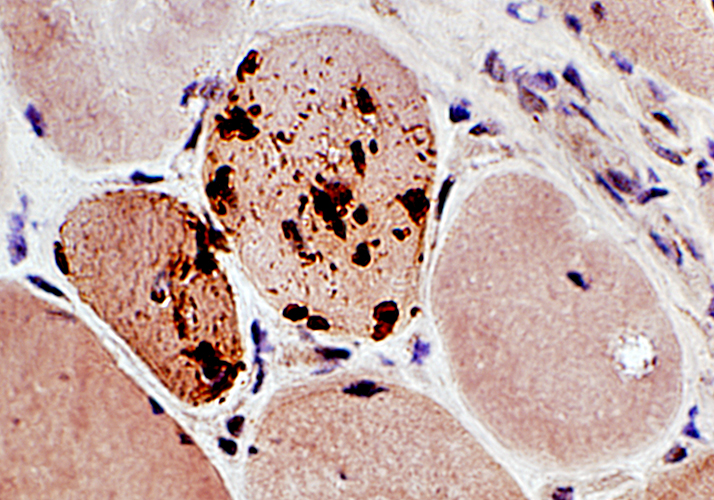 SMI-31 + Congo red stains |
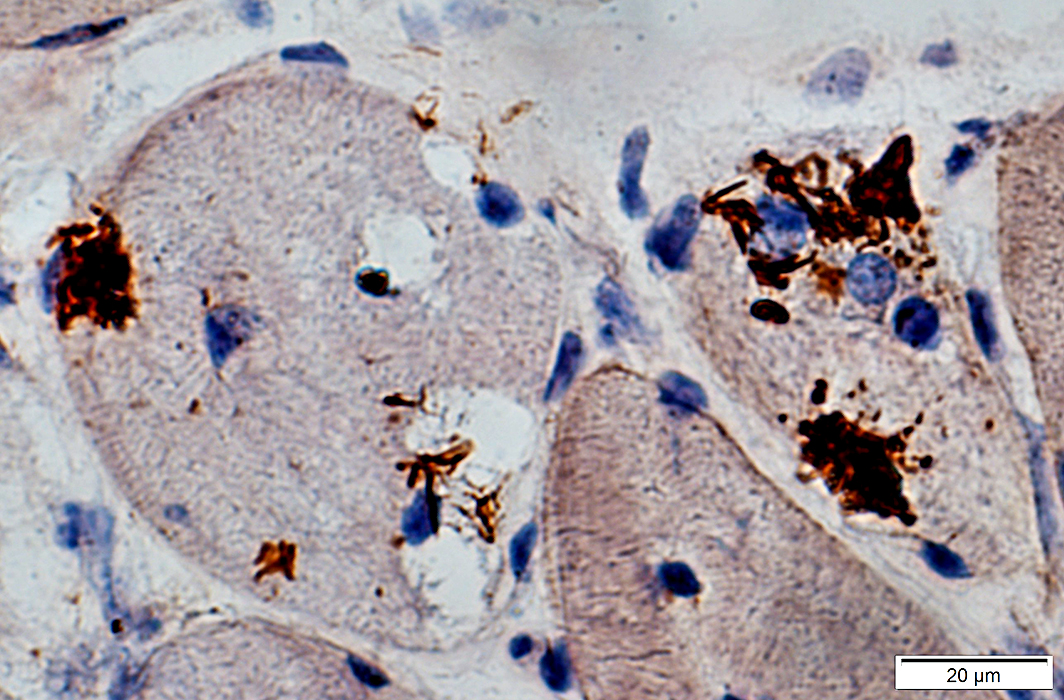 SMI-31 + Congo red stains |
SMI-31 staining in some nuclei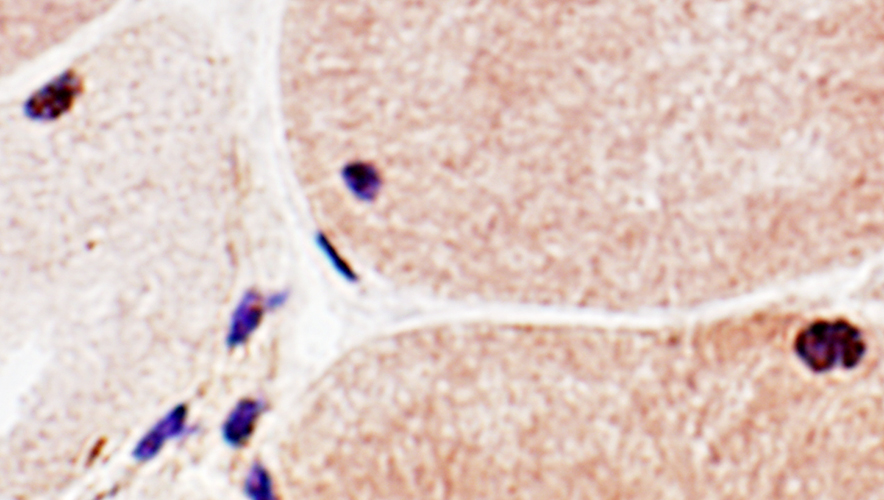 SMI-31 + Congo red stains |
|
|
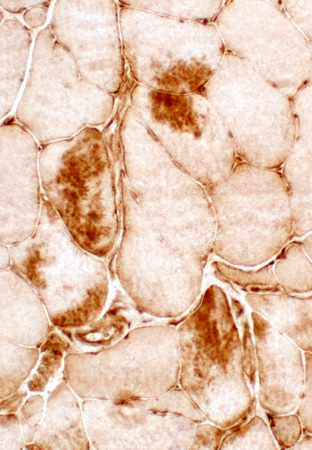
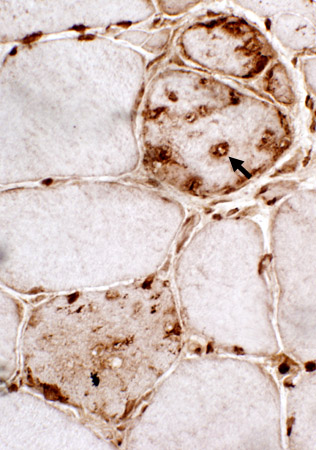
αB-crystallin
|
|
|
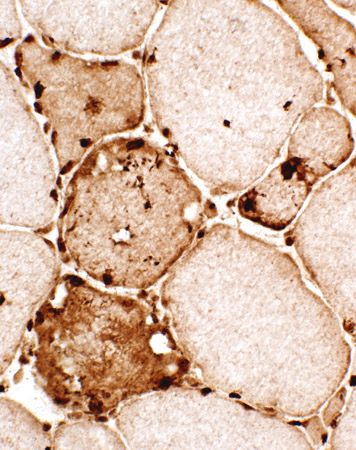
|
Ubiquitin conjugates: IBM
|
|
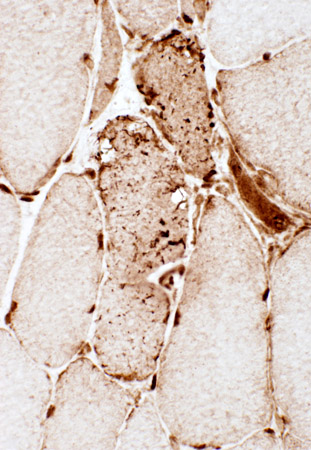
|
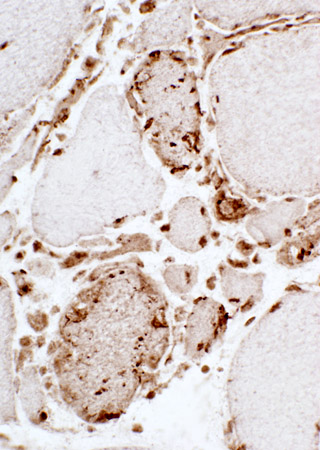
|
Valosin-containing protein (VCP): IBM
|
|
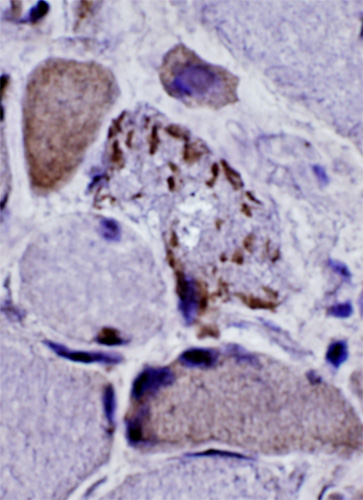 Congo red + β-amyloid stain |
 Congo red + fluorescence |
β-Amyloid: IBM
|
|
TDP-43 aggregates
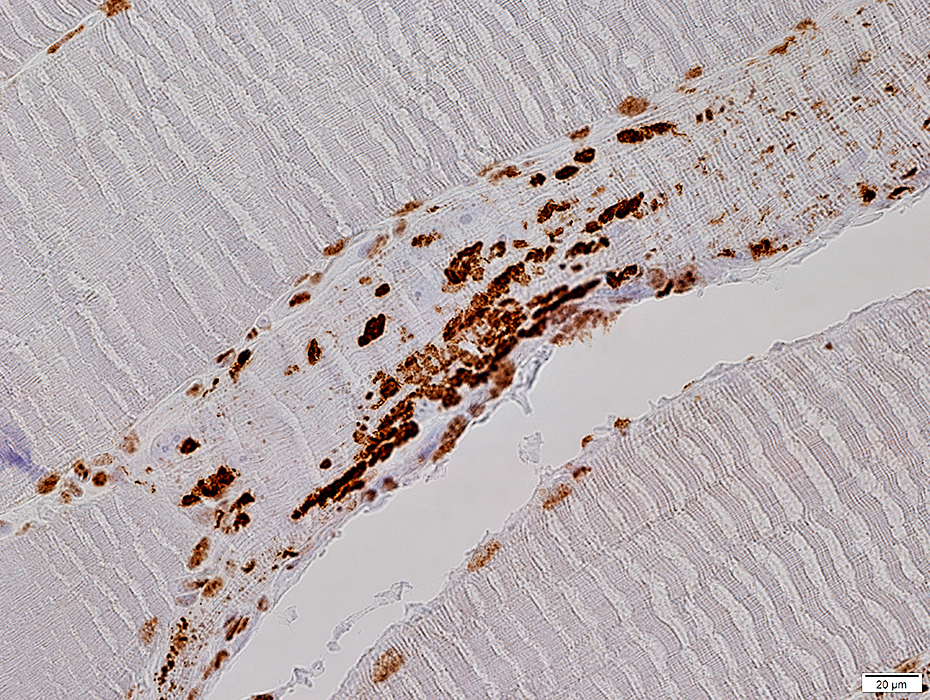 TDP-43 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
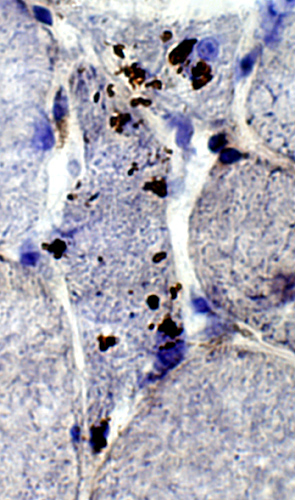
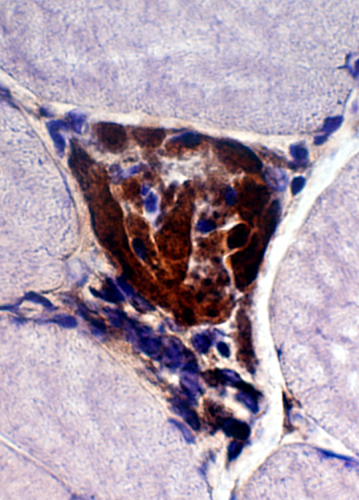 Congo red + TDP43 |
|
TDP-43: IBM
|
|
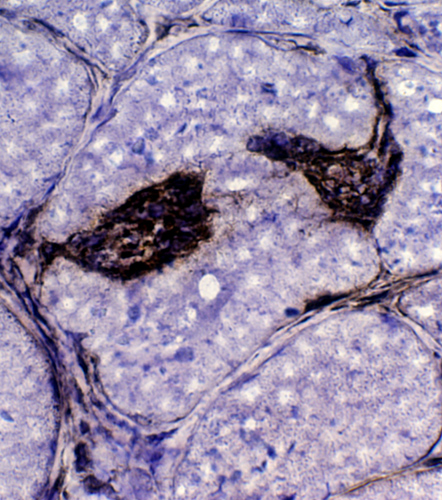
 Congo red + TDP43 |
|
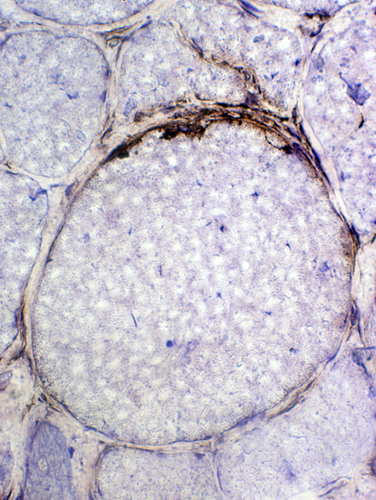
 CD4 (Green) + TDP43 (Red) TDP-43: Aggregates & Inflammatory cells in IBM
|
|
 SMI-31 (Green) + TDP43 (Red) TDP-43: Aggregates and cytoplasmic staining with varied relation to SMI-31 staining
|
|
p62 aggregates
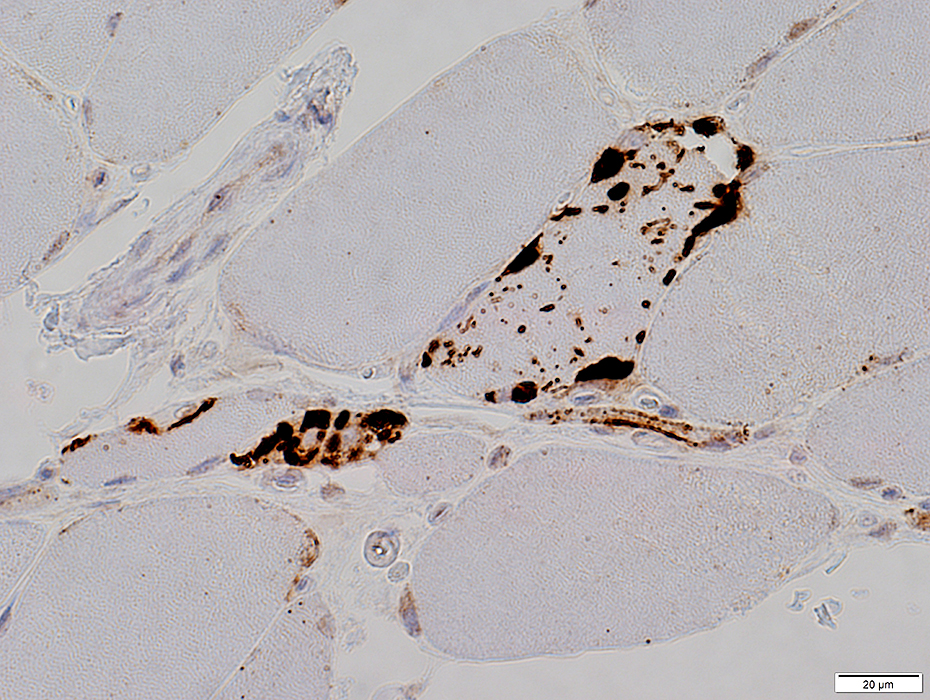 p62 stain |
 p62 stain |
LC3 Aggregates
|
Differential Diagnosis sIBM/IM-VAMP syndromes Immune myopathies Dermatomyositis + Vascular pathology Tif1-γ IMPP MDA5 Systemic Sclerosis Hereditary Myopathy LGMD1A MSP OPMD Lipid disorders Myosin loss Targets |
LC3 aggregates: IM-VAMP (IBM-like syndromes)
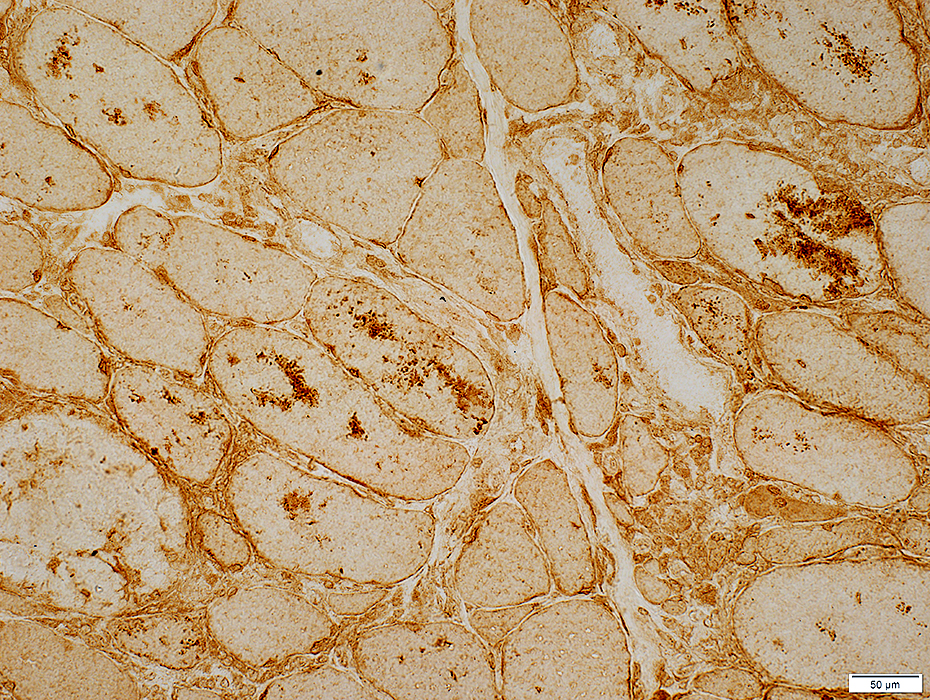 LC3 stain |
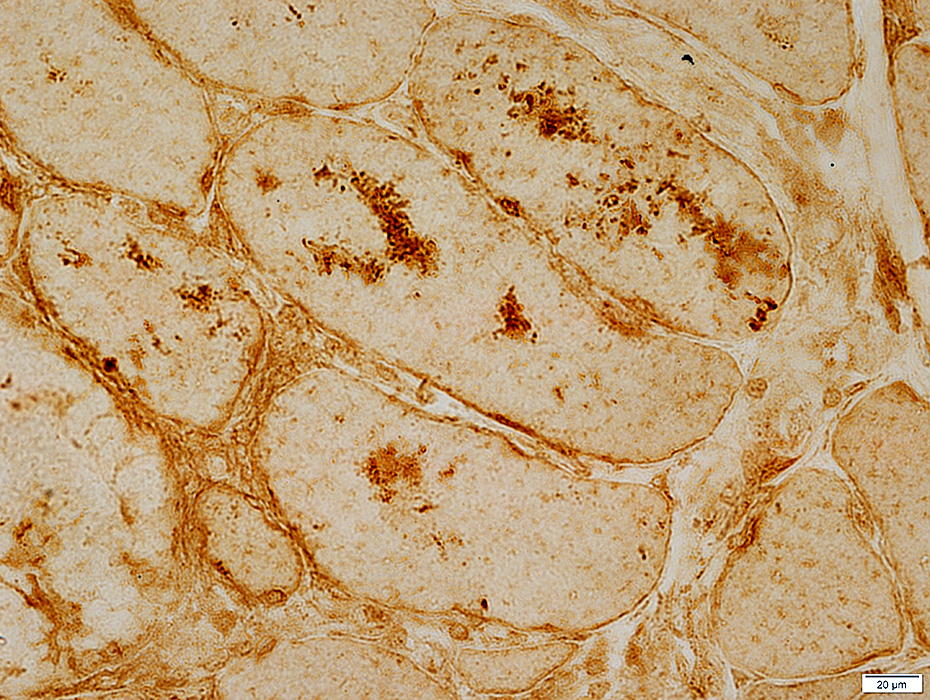 LC3 stain |
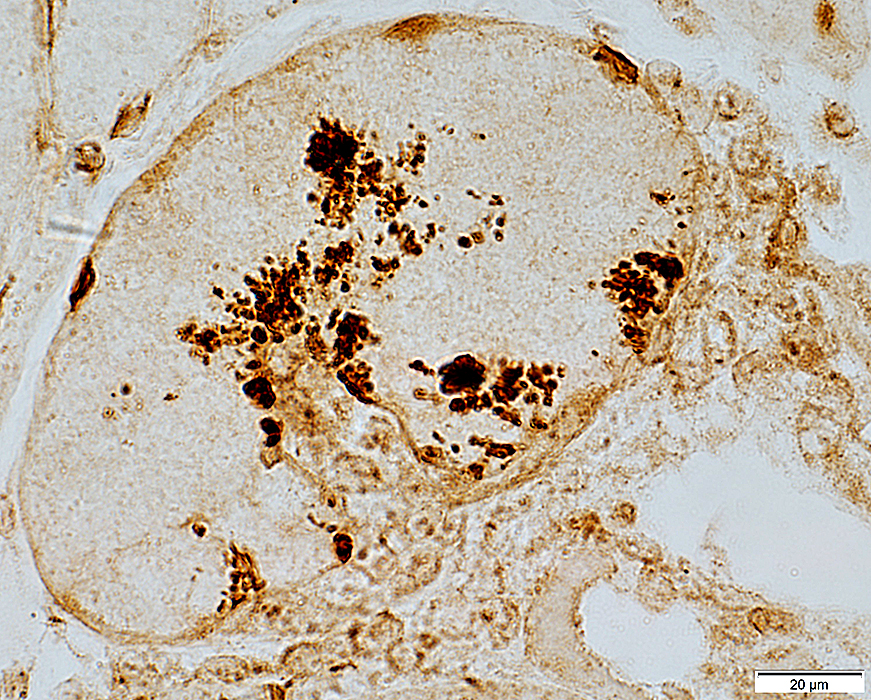 LC3 stain |
- Focal regions of dark staining of varied shapes (Left)
- Aggregates are cytoplasmic, small or large & have varied shapes
- LC3 aggregates are also present in PM-Mito biopsies (Right), unlike SMI-31 or TDP-43
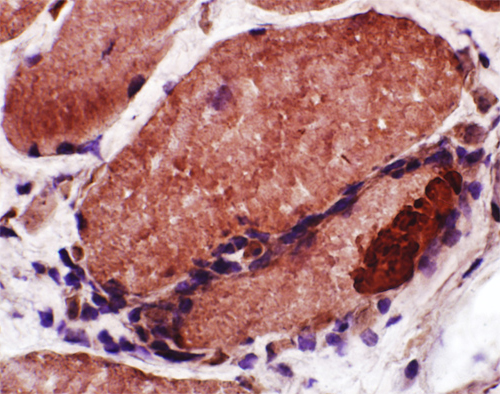 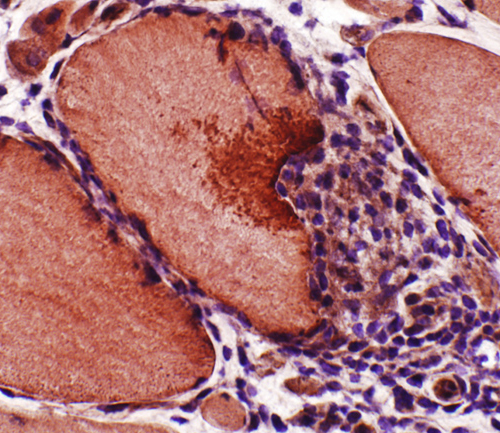 |
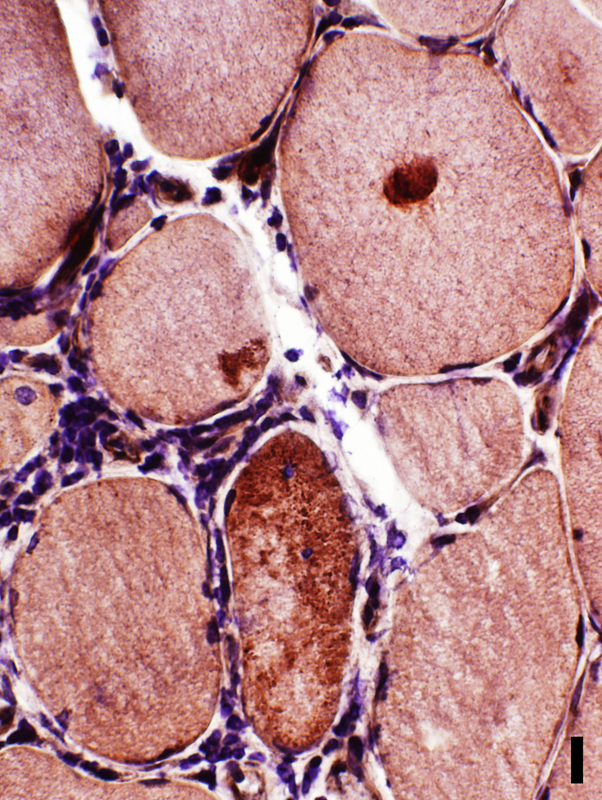 Congo red + LC3 stain |
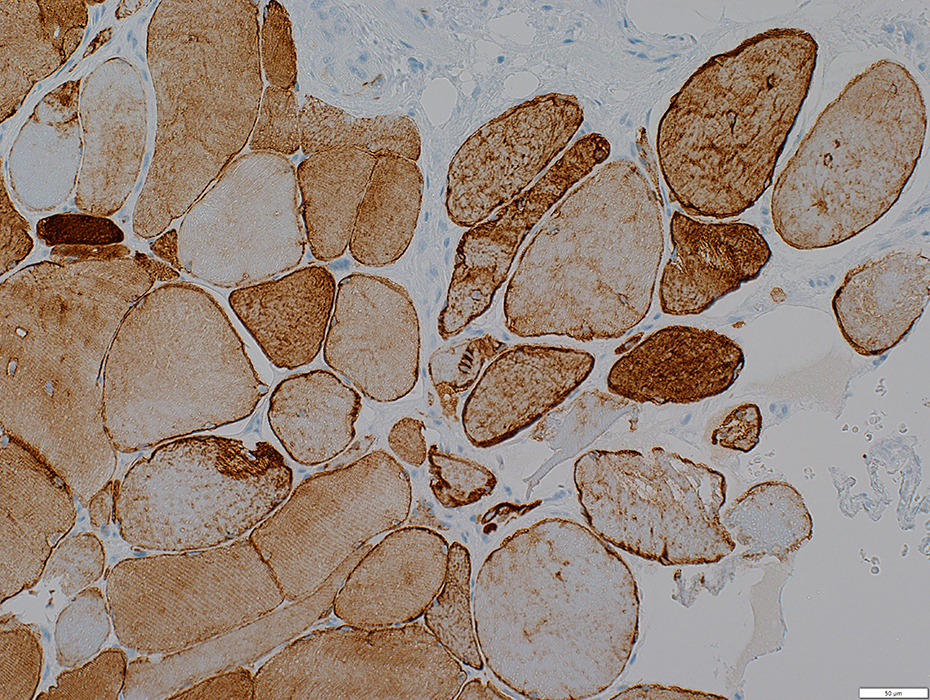 Desmin stain |
Diffusely in cytoplasm of iommature muscle fibers
Irregular aggregates in scattered muscle fibers
Localized at site of focal invasion of a muscle fiber (below)
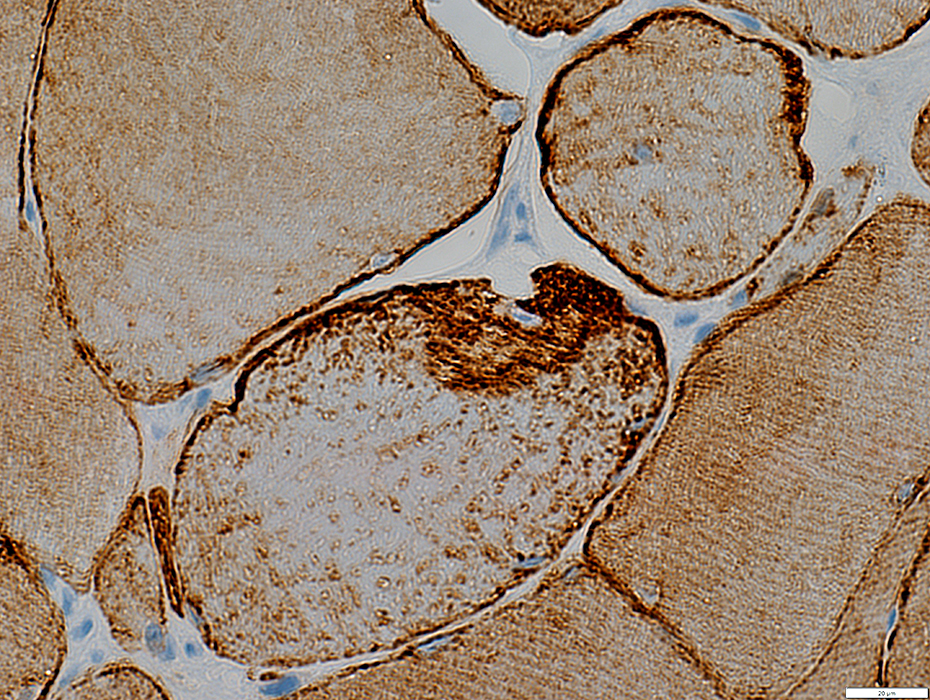 Desmin stain |
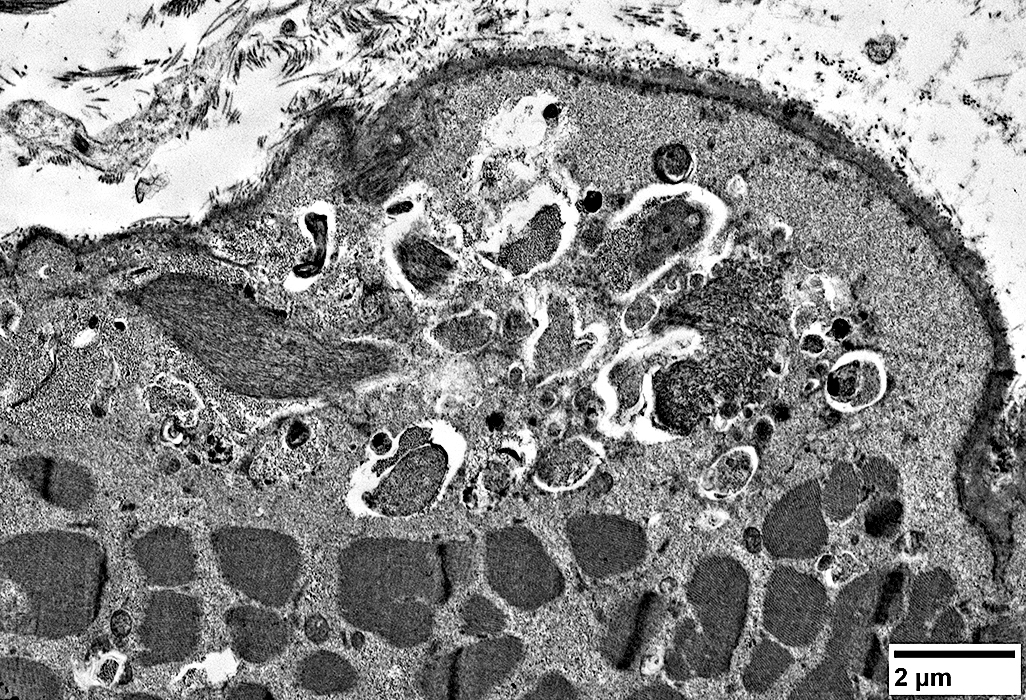
IM-VAMP: Ultrastructure
|
Focal invasion of muscle fibers by cells Aggregates & Inclusions, Cytoplasmic Autophagic Vacuoles |
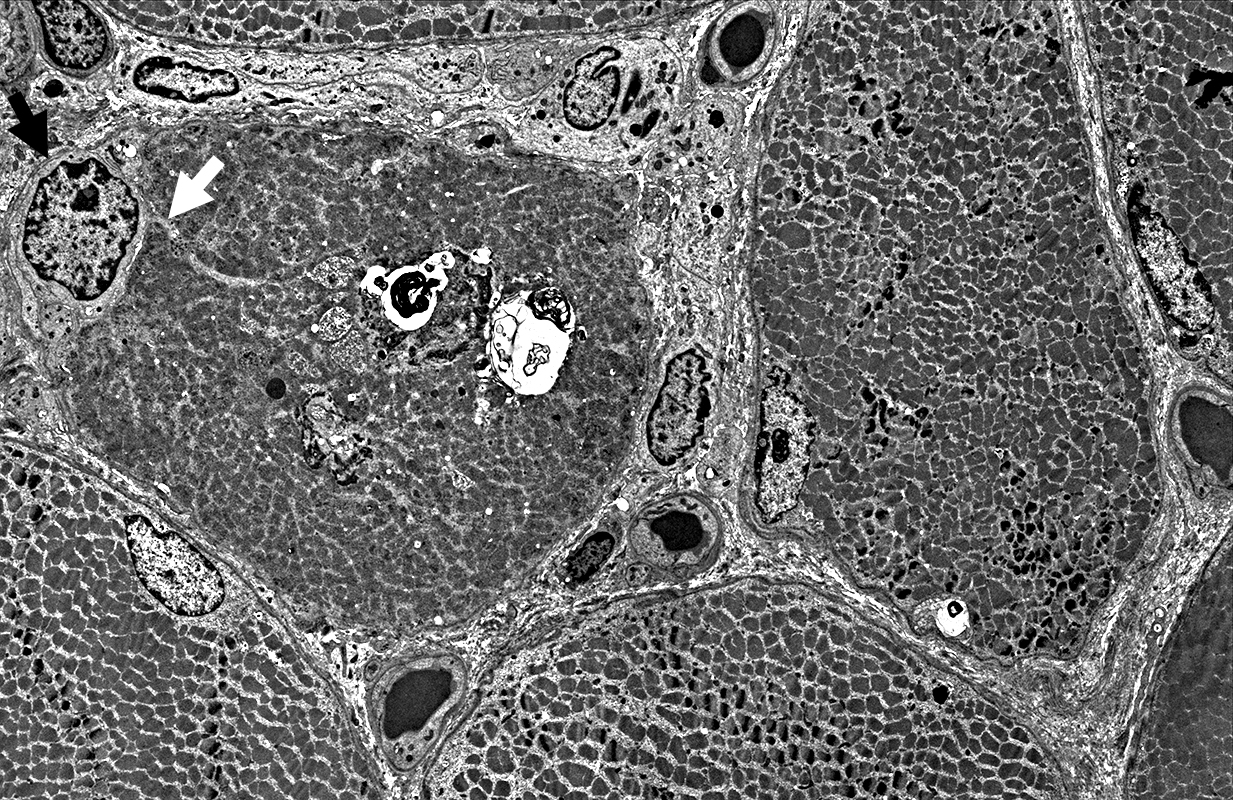 |
Cytoplasmic inclusions: Several types
Myeloid
Membrane-like
Tubulo-Vesicular
Filamentous
Autophagic debris
Lipid-like bodies
Mitochondria
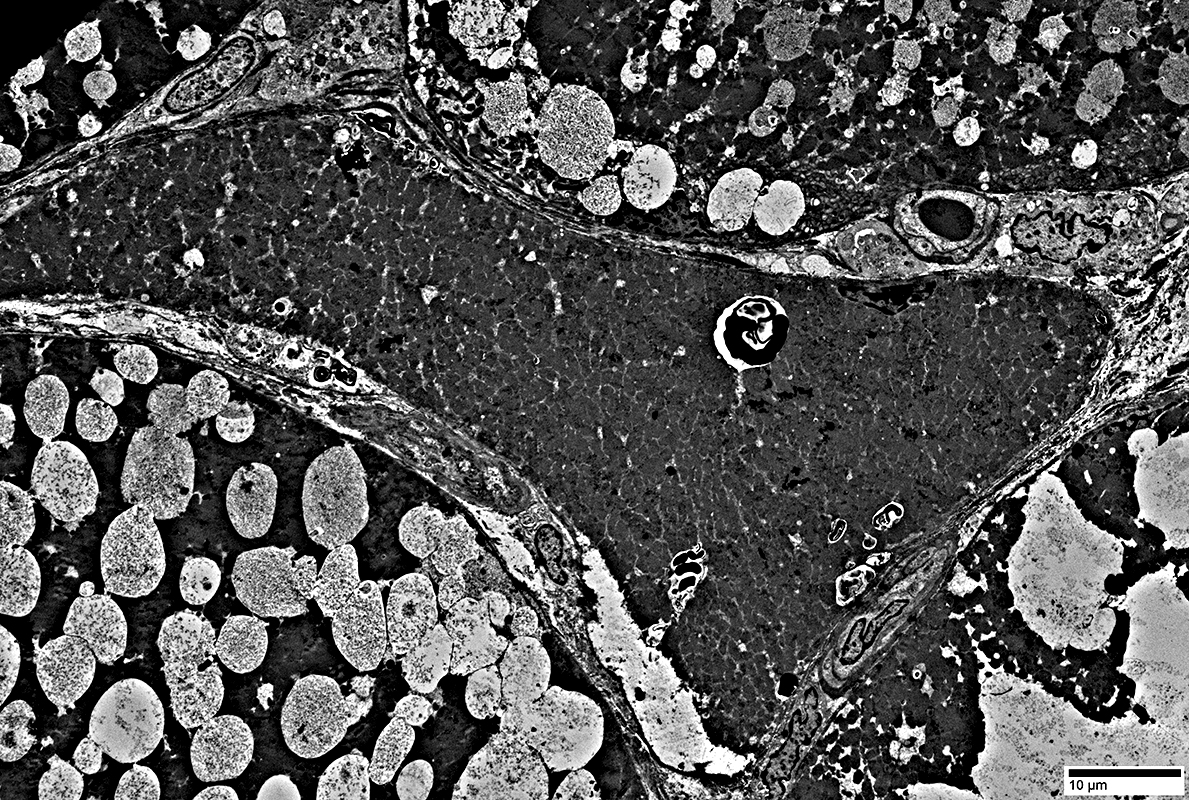
Endomysial capillaries
Normal size & walls
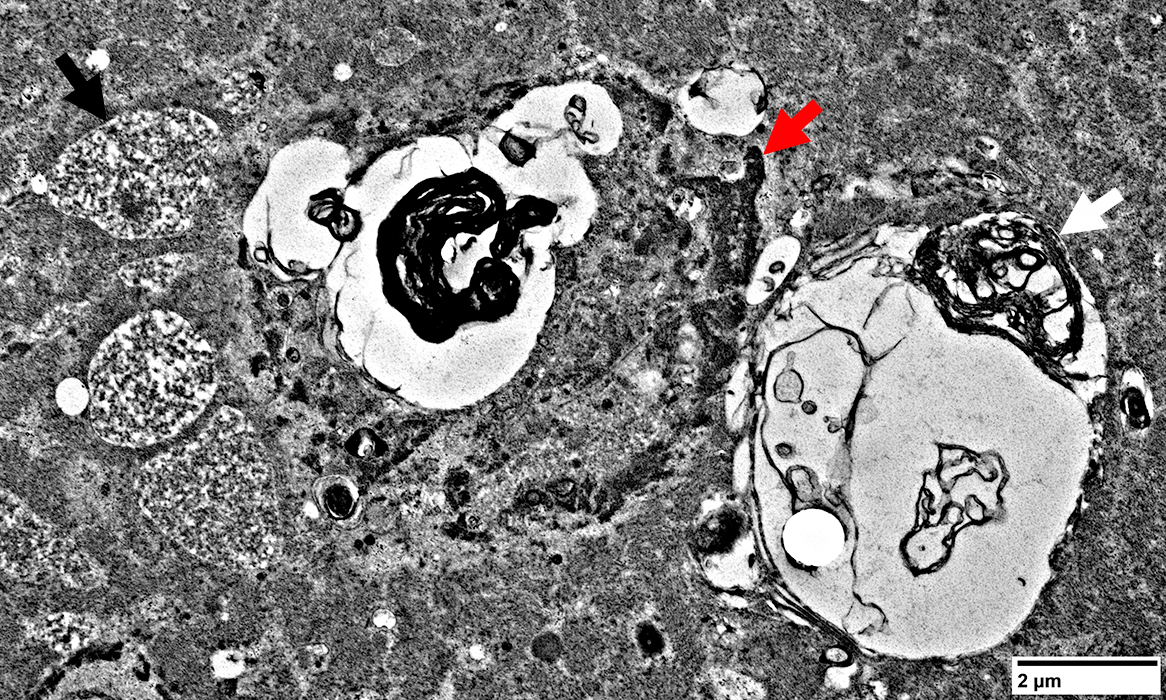 |
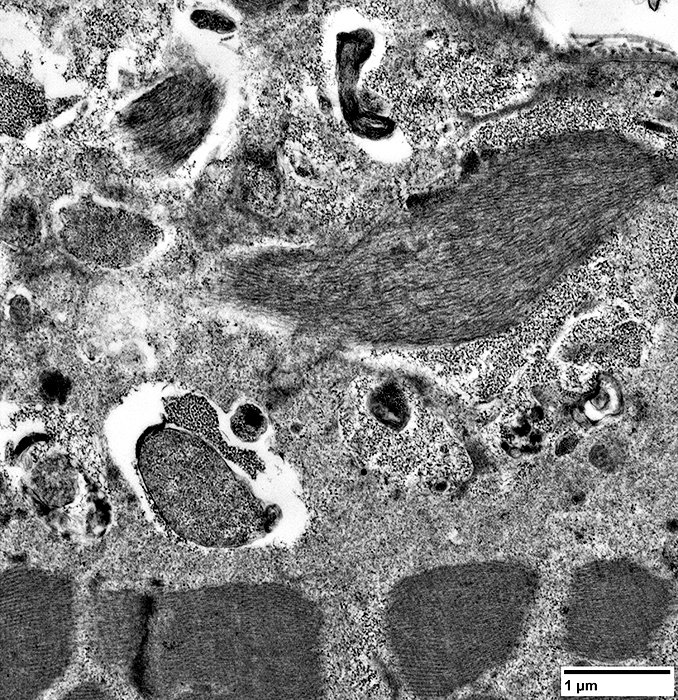
- Filamentous (Black arrow; Above)
- Osmophilic (Red arrow; Above): Possible remanants of nuclear membranes
- Autophagic
- Membrane-like (White arrow; Above): Multilayered; Irregular shapes; May be within larger, "vacuolar", structures
- Tubulovesicular material (Below)
- Membrane-like (White arrow; Above): Multilayered; Irregular shapes; May be within larger, "vacuolar", structures
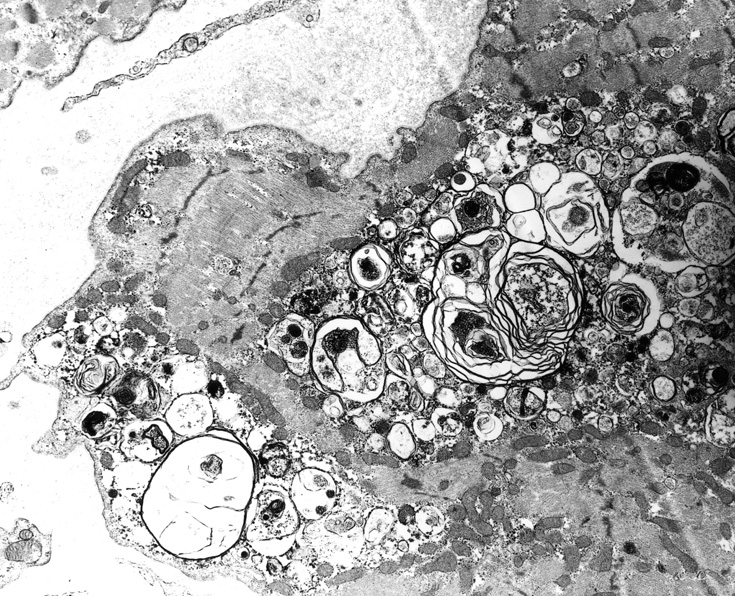 From: R Schmidt |
Autophagic Vacuoles
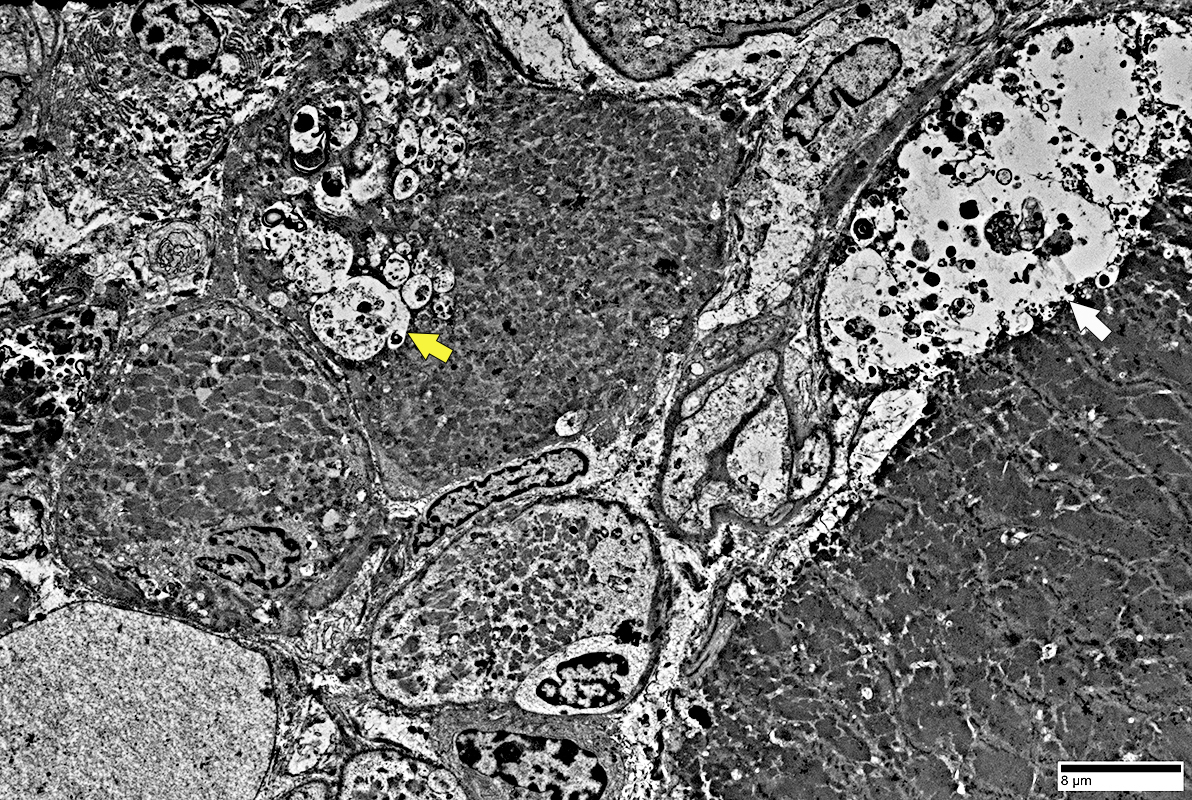
|
Multiple small vacuoles in small muscle fiber (Yellow arrow)
Large autophagic vacuole: Budding from the surface of a large muscle fiber (White arrow)
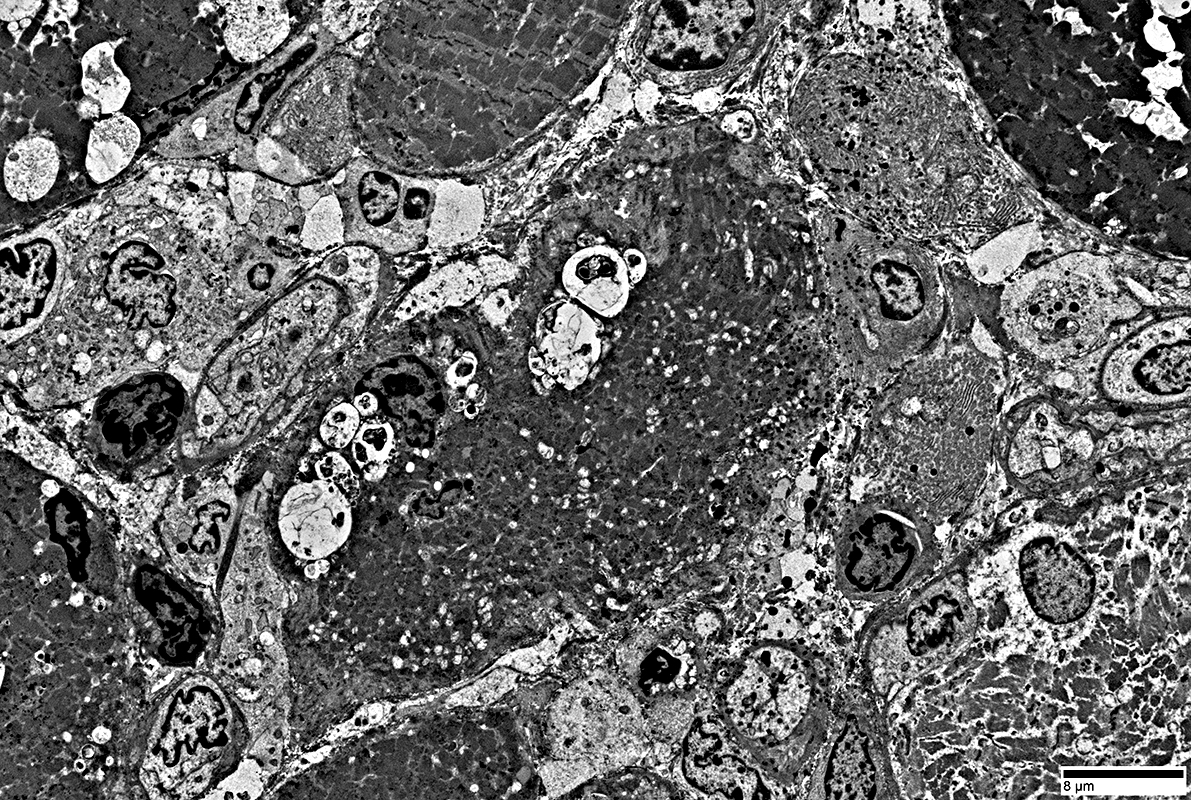
|
Multiple small vacuoles in small muscle fiber
Lymphocytes
Location: Endomysium; Surround muscle fiber
|
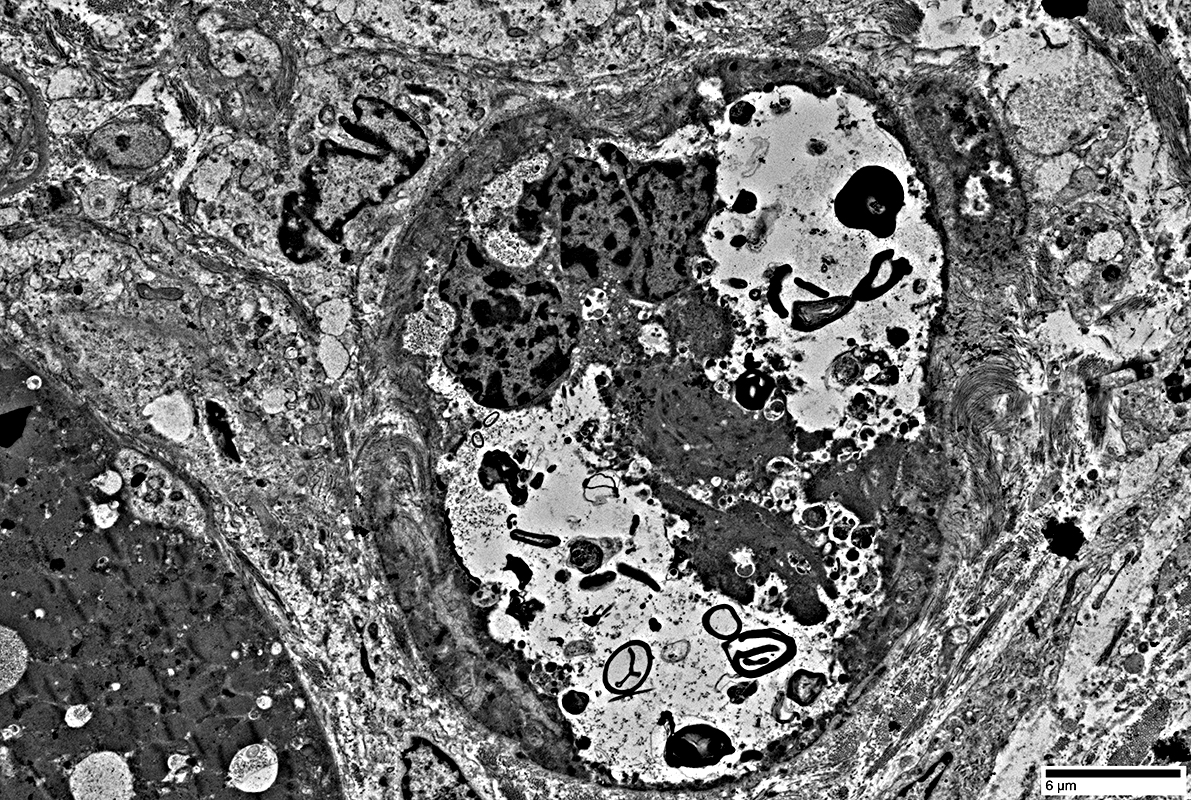
Large vacuole replacing most cytoplasm in small muscle fiber (Above)
Contents of vacuole (Below)
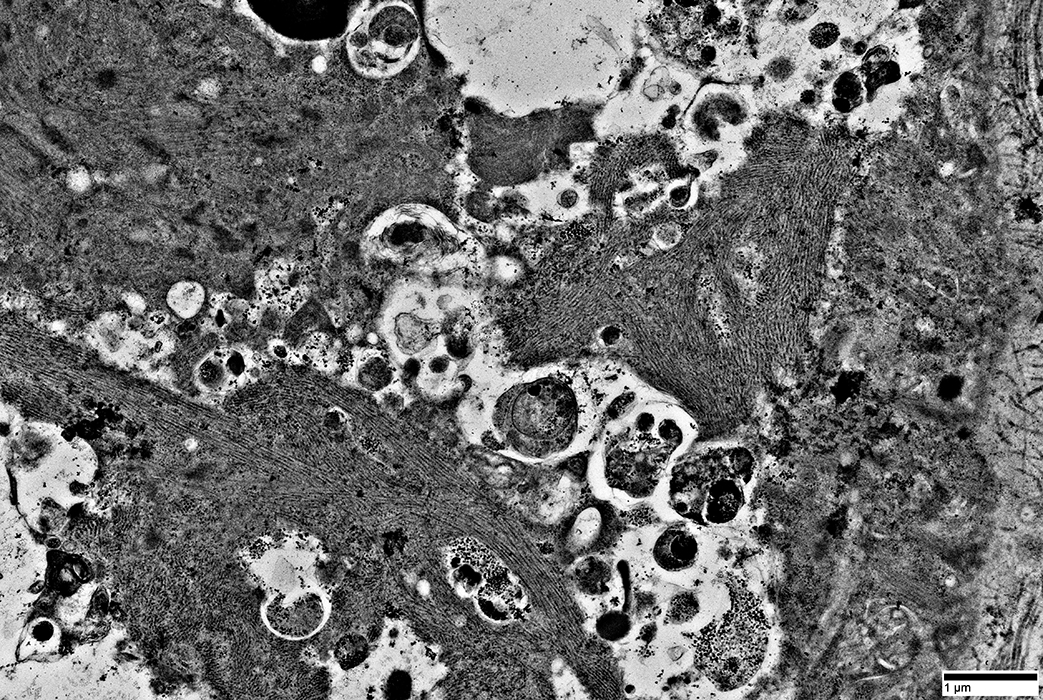
|
|
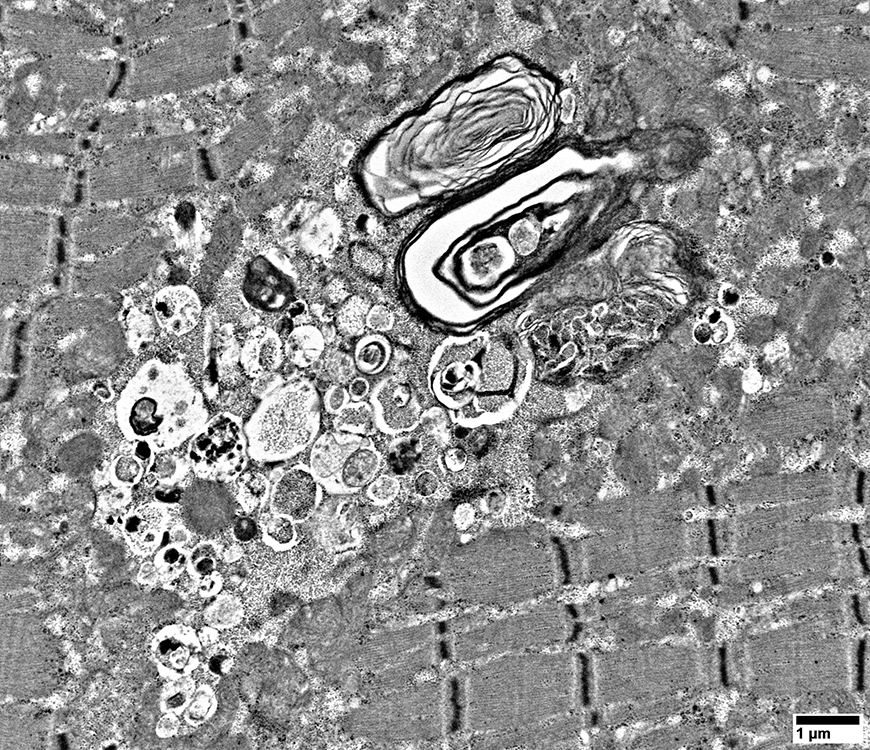
Filaments: Varied densities
Myeloid figures
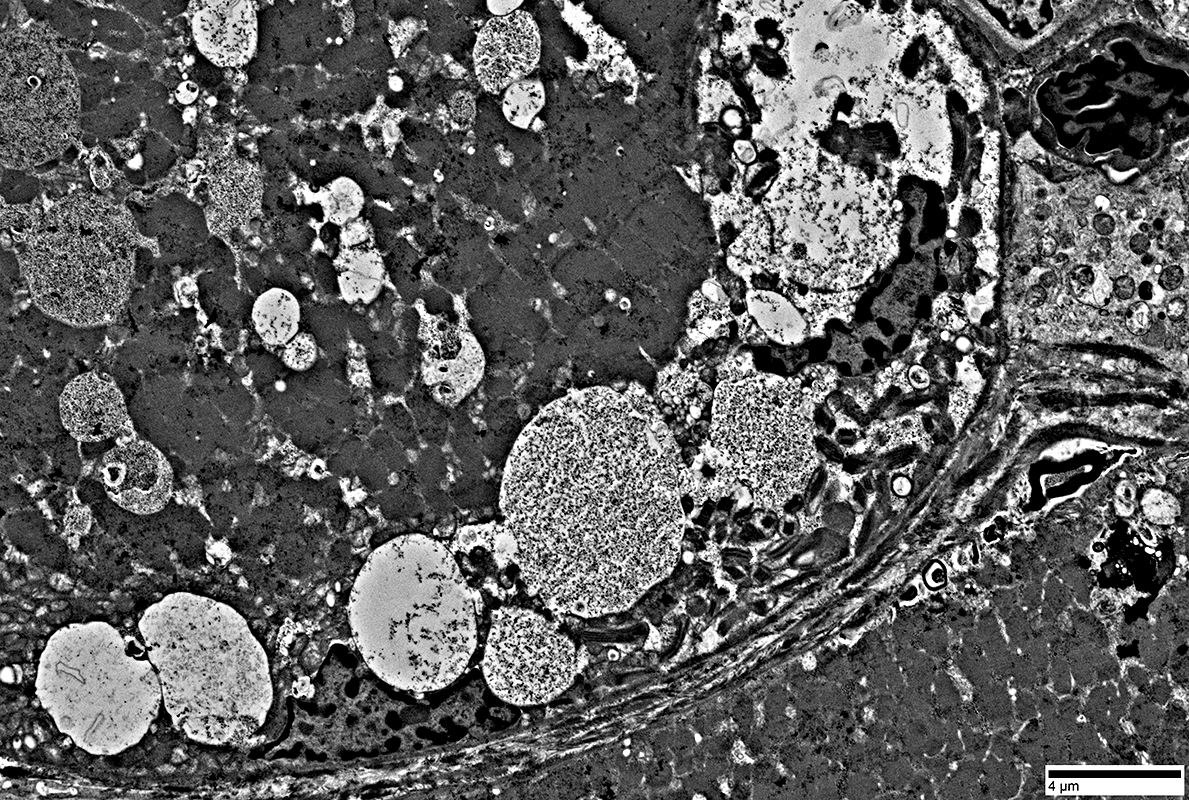
|
Aggregates
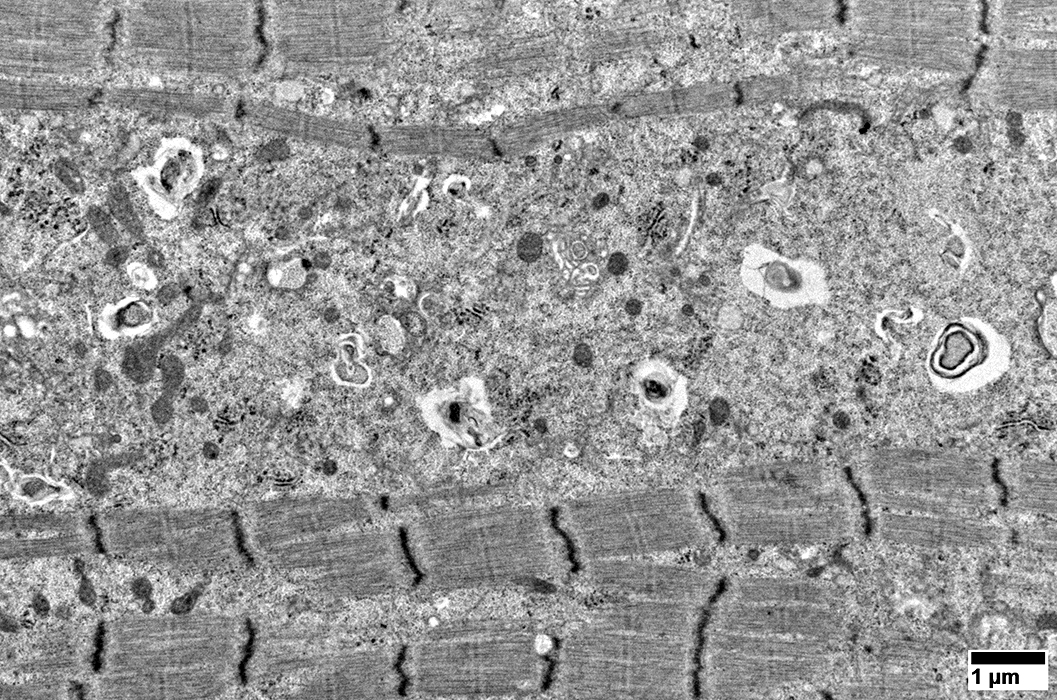 From: R Schmidt |
Filaments
A few myeloid structures & mitochondria
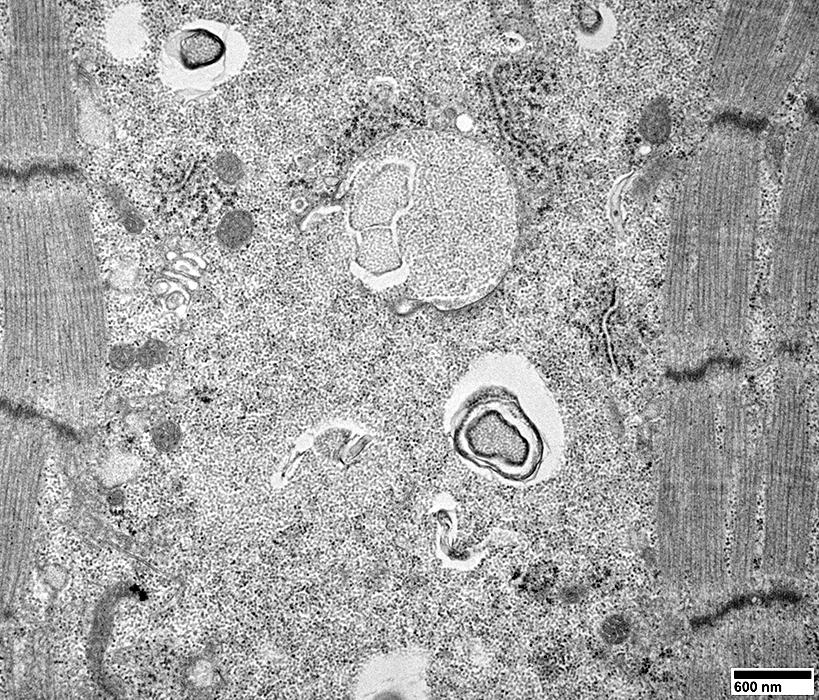 From: R Schmidt |
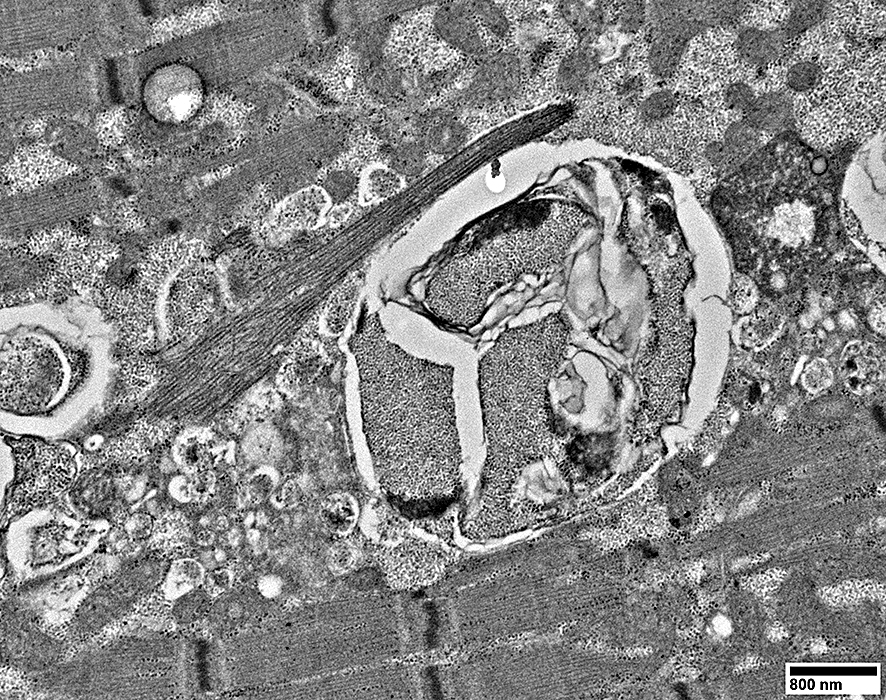
Filaments
A few myeloid structures & mitochondria
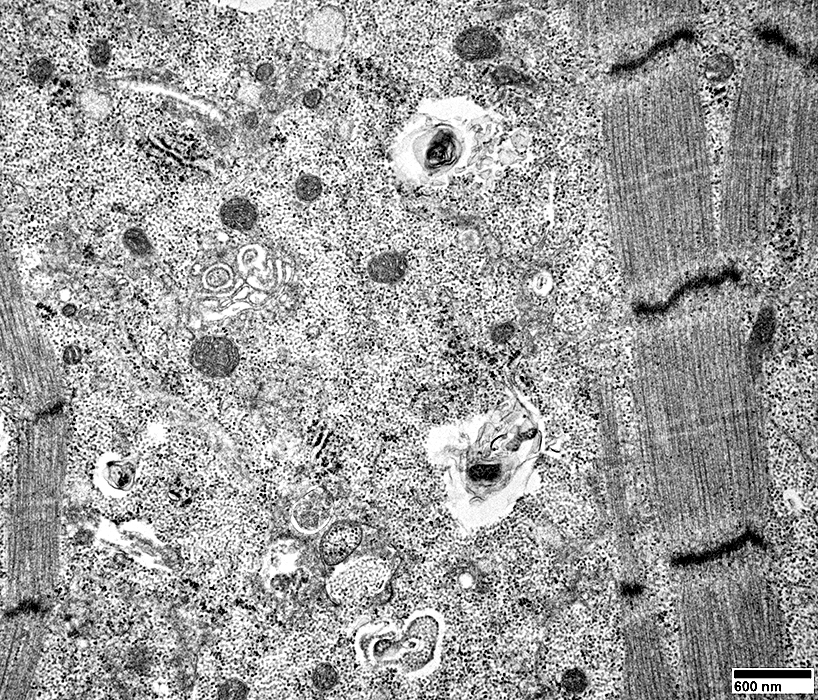 From: R Schmidt |
 |
Filaments
Myeloid structures
 |
|
|
 |
Filaments
Myeloid structures
Mitochondria
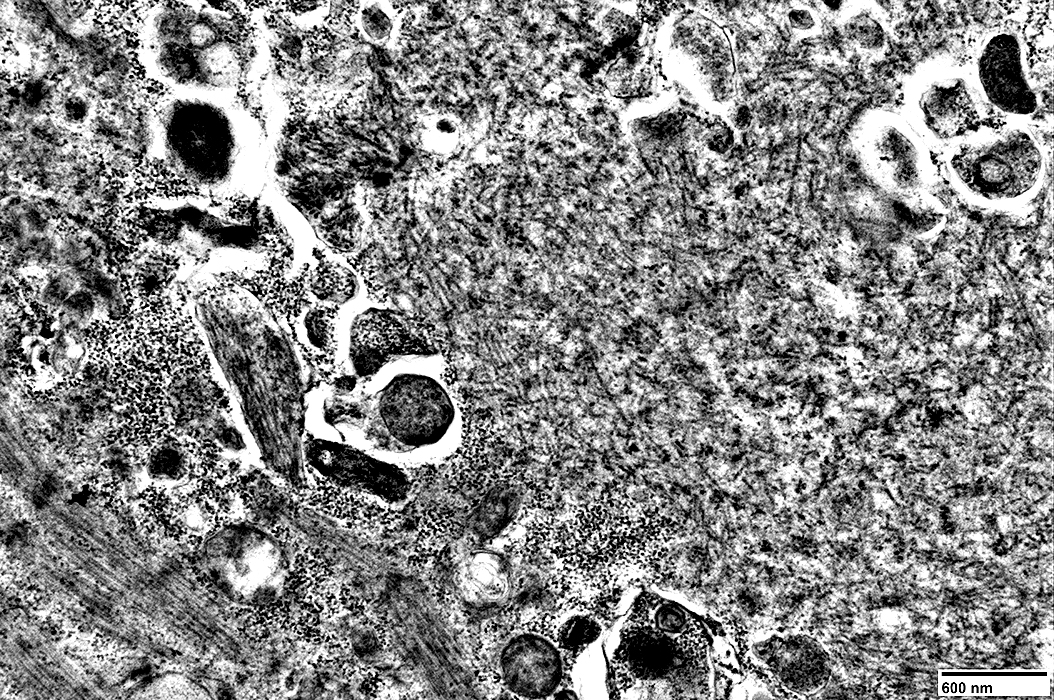 |
 |
Mitochondria
 |
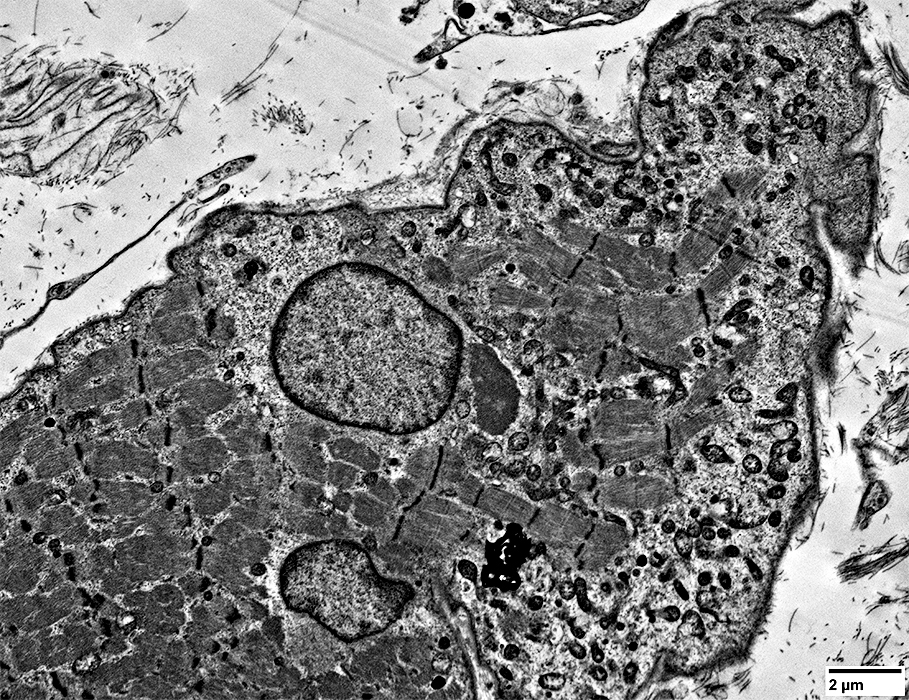
Muscle fibers: Focal Invasion by Cells
 |
Lymphocyte, Large, Possibly Granular (Black Arrow)
Extends a process (White arrow) into a non-necrotic muscle fiber
Other lymphocytes & histiocytes
Whole immune cells may be invading muscle fiber
Present in extracellular regions around muscle fiber
Muscle fiber pathology
Aggregates, various types
Vacuoles
 |
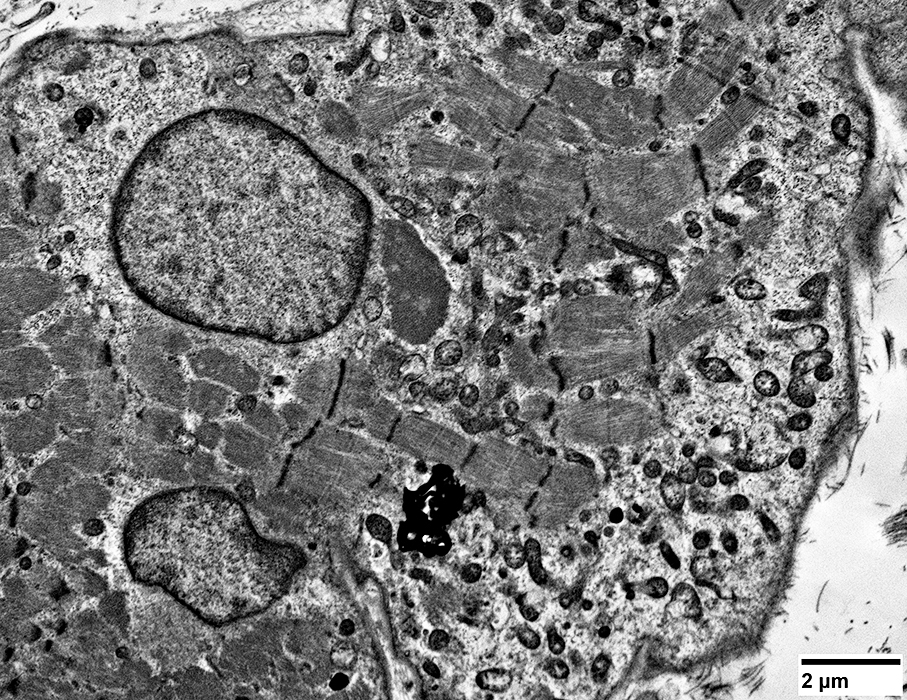
Lymphocyte, single, invades muscle fiber
Lymphocyte location: Inside muscle fiber; Above sarcolemma; Neighbors myonucleus
 |
Lymphocytes (Light cytoplasm) & Histiocytes (Dark cytoplasm with phaogcytic debris)
Within the muscle fiber
Closely apposed to each other
Also in extracellular space
Sarcomeres near invading cells: Normal structure
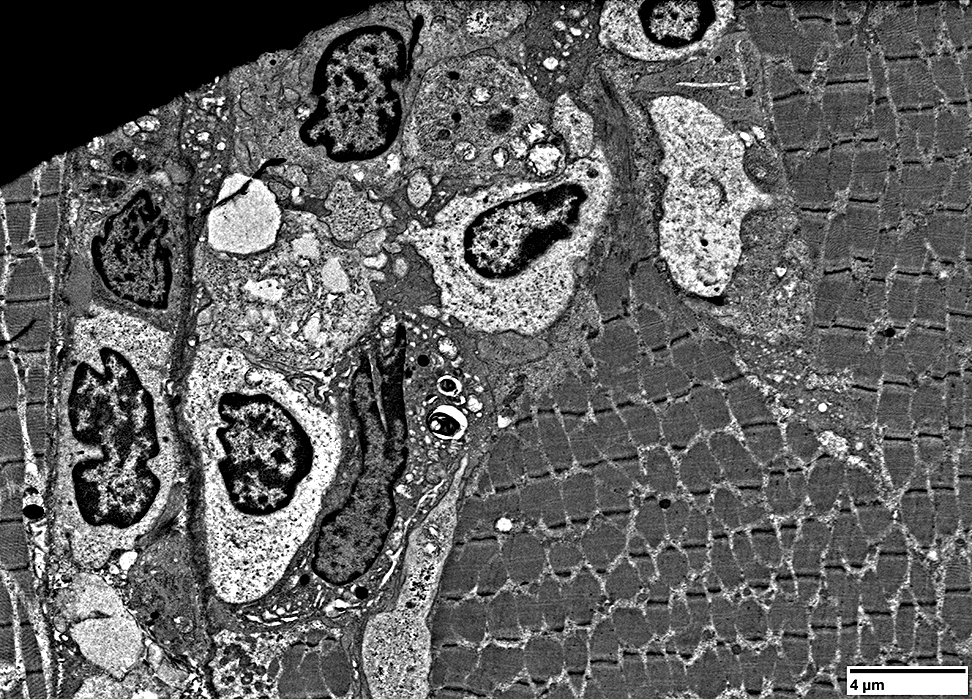
Muscle fiber: Focal Invasion by small cell processes
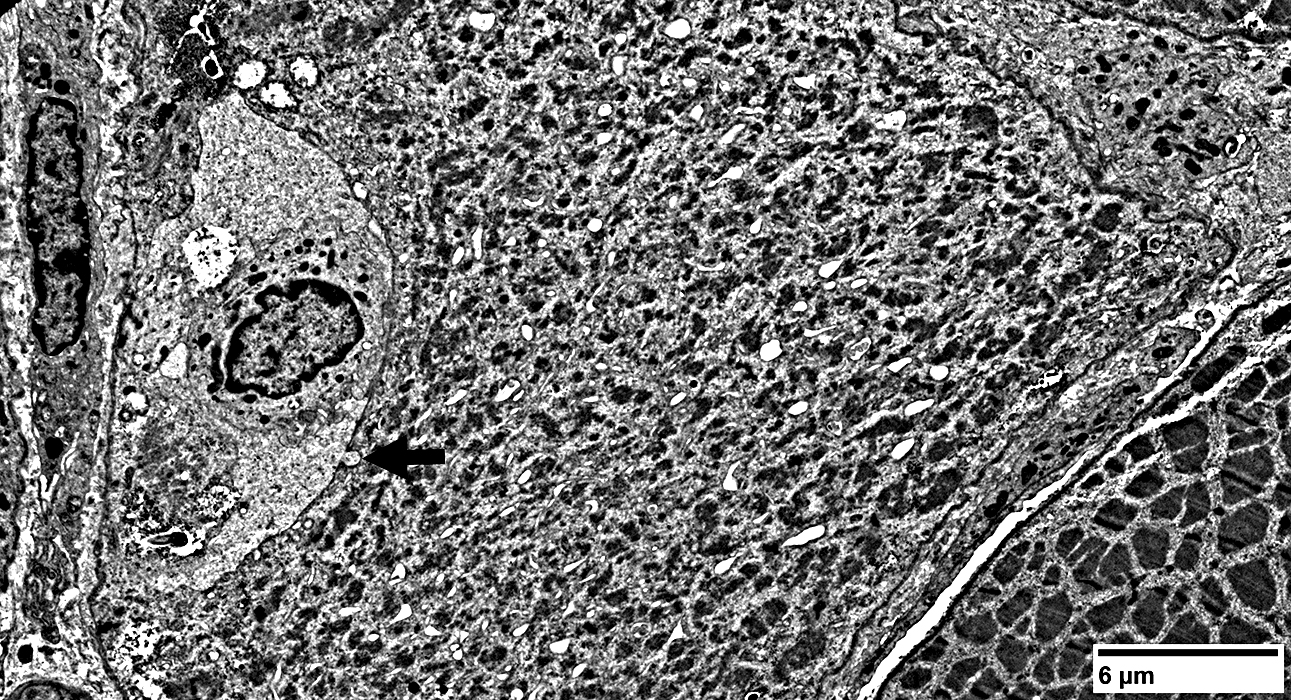 |
Muscle fiber structure is damaged
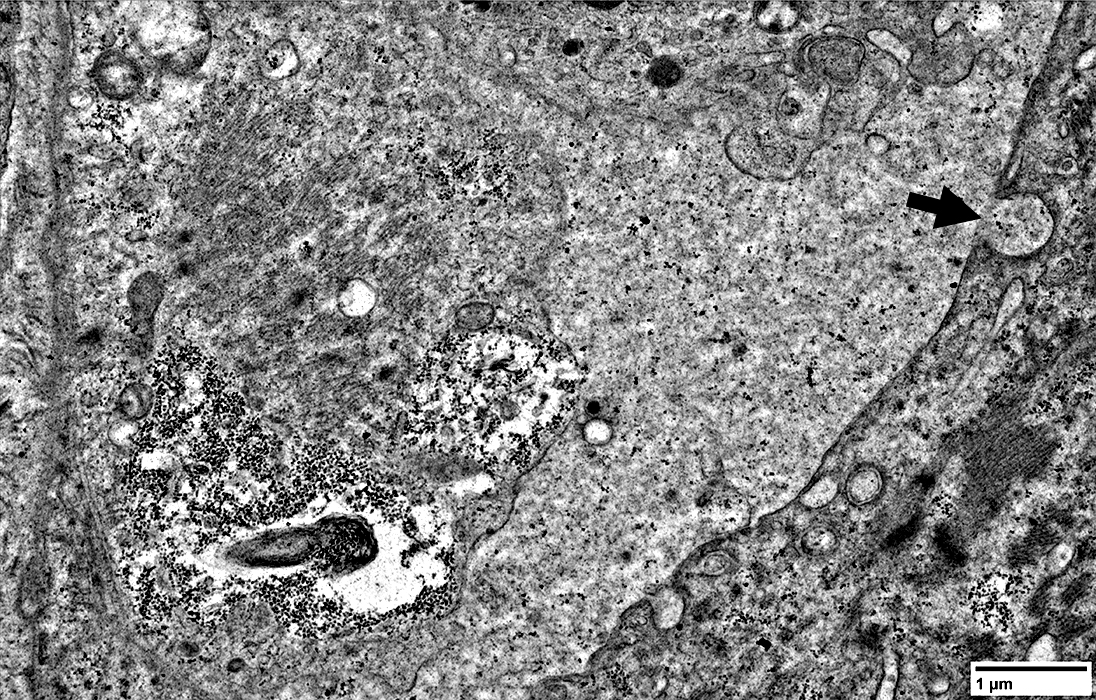 |
MHC-1 Expression
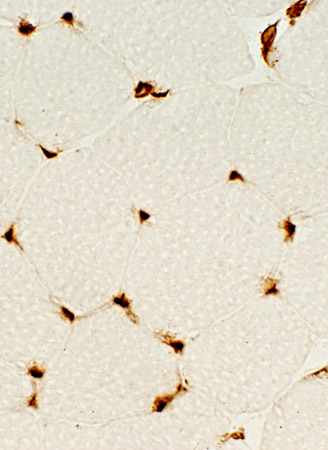 Normal |
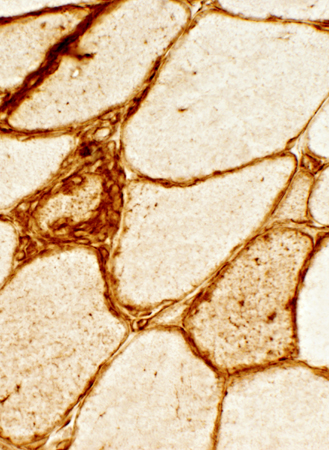 IBM |
- Normal (Left)
- Staining of capillaries but not muscle fibers
- IBM (Right & Below)
- Up-regulation within, and on the surface of, muscle fibers.
- MHC-1 is also present on inflammatory cells, Some focally invading muscle fibers (Arrow)
- Immature, smaller fibers can also express MHC Class-1 on their surface & in cytoplasm
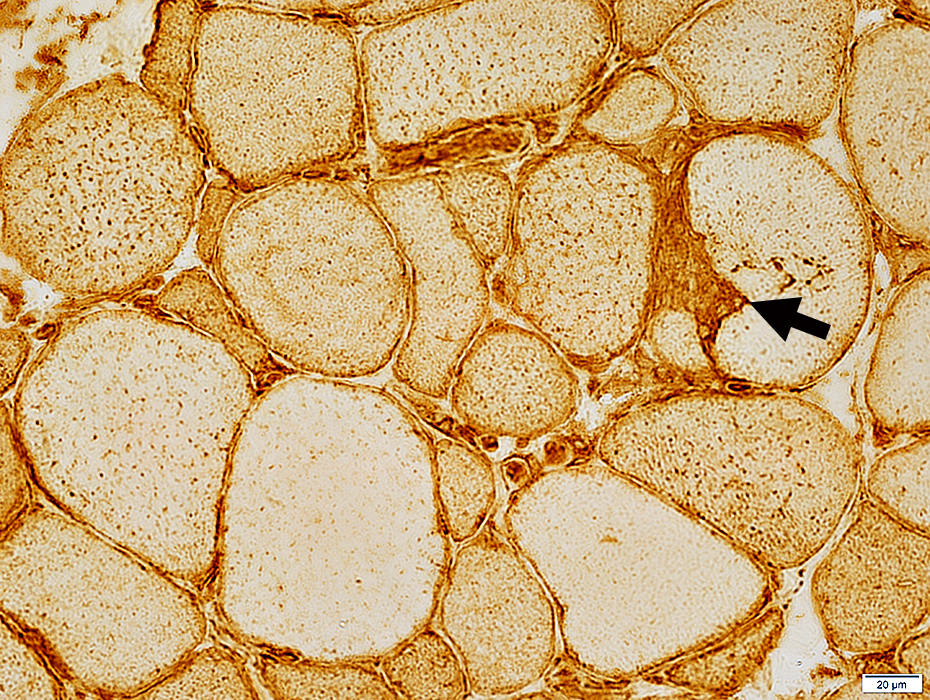 MHC Class I stain |
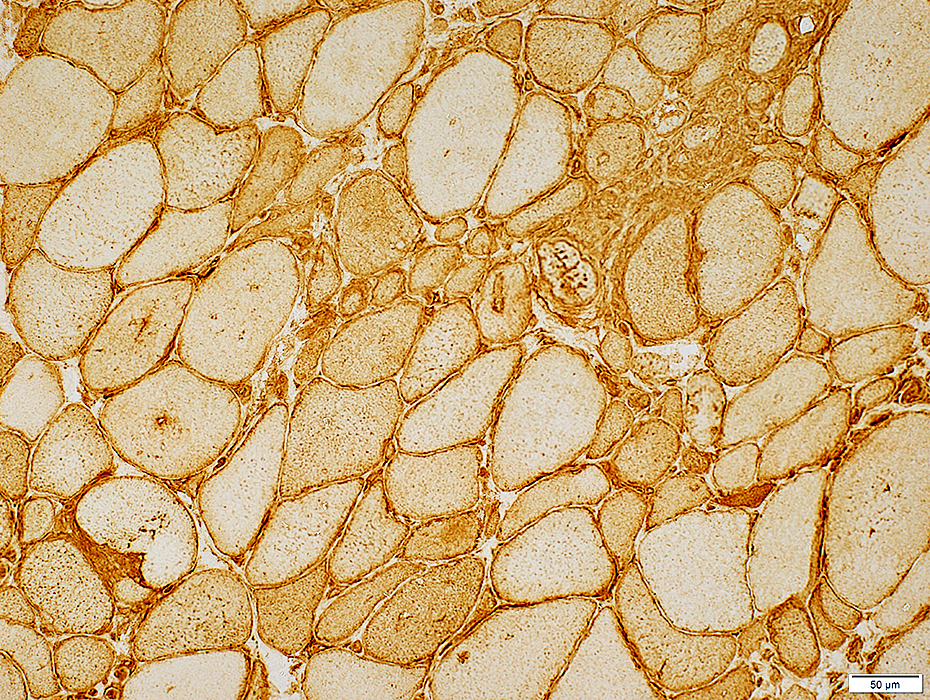 MHC Class I stain |
Diffuse: Present in most, or all, muscle fibers
Distribution
Mostly Sarcolemma, or Sarcolemma + Cytoplasm
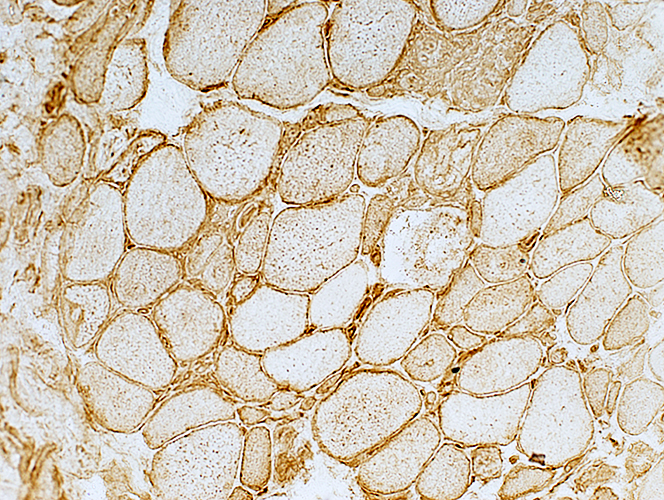 MHC Class I stain |
Also see
IM-VAMP: Myonuclear Pathology
IM-VAMP: Nuclear features
Location: Internal nuclei
Structure
Clear centers
Outlines: Smudged or Pale
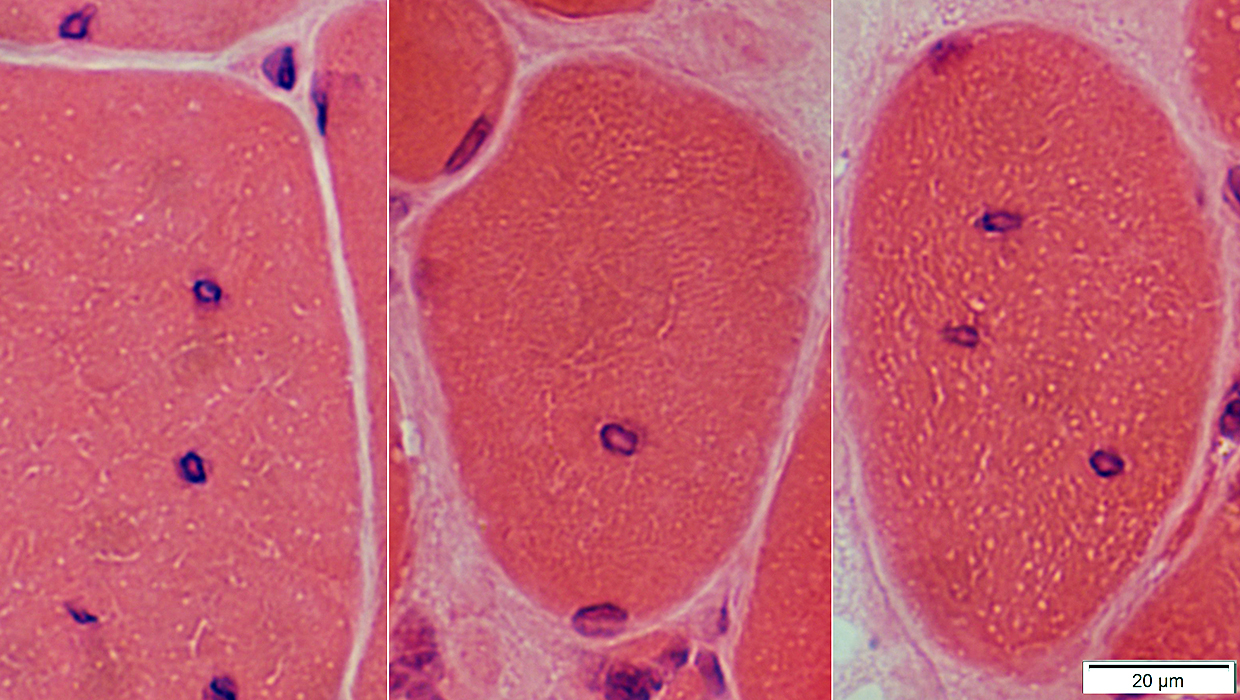 H&E stain |
Internal: One or Several
Structure: Clear centers
May have peripheral cytoplasmic halo
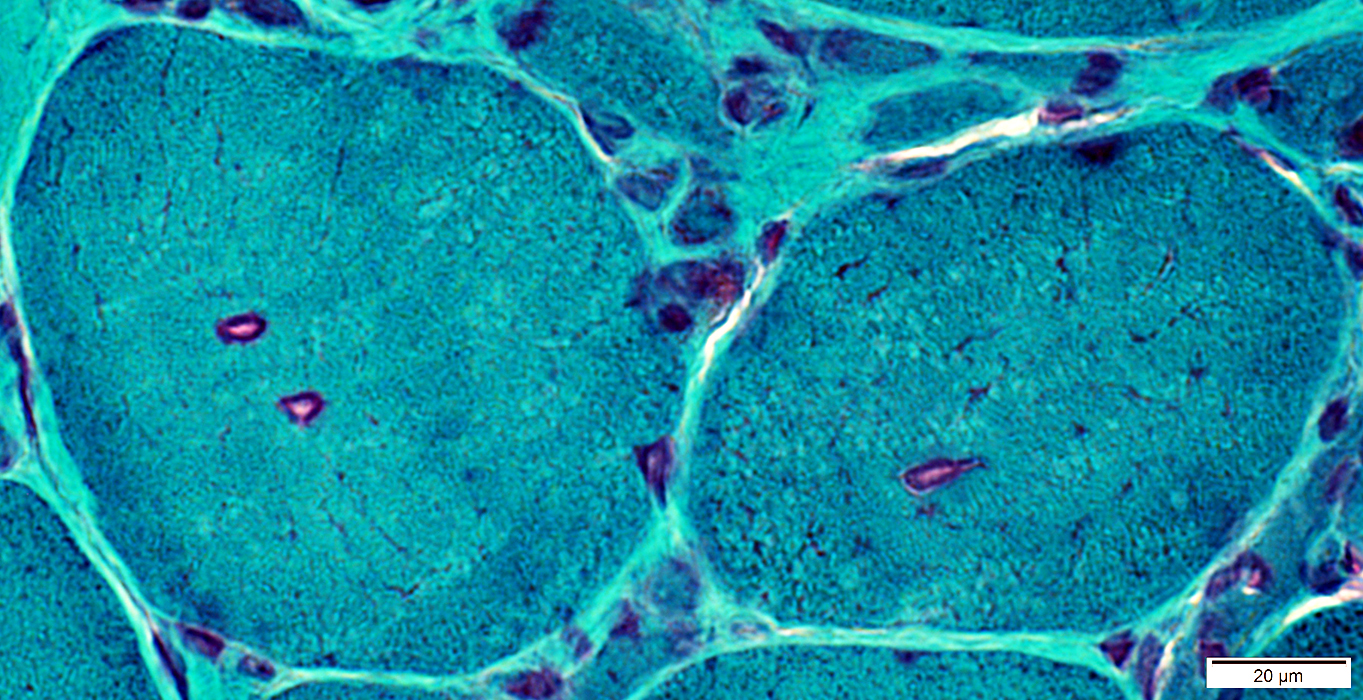 Gomori trichrome stain |
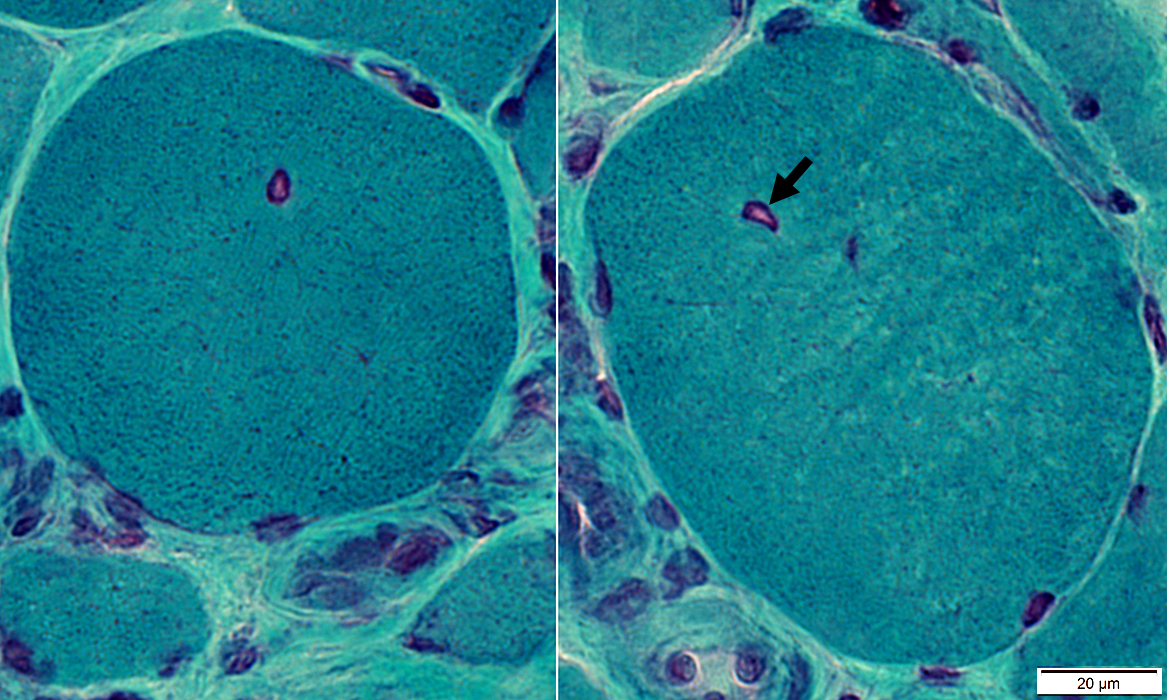 Gomori trichrome stain |
IM-VAMP Myonuclei: Smudged outlines or Clear centers
 Congo red stain |
Indistinct borders
Irregular shapes
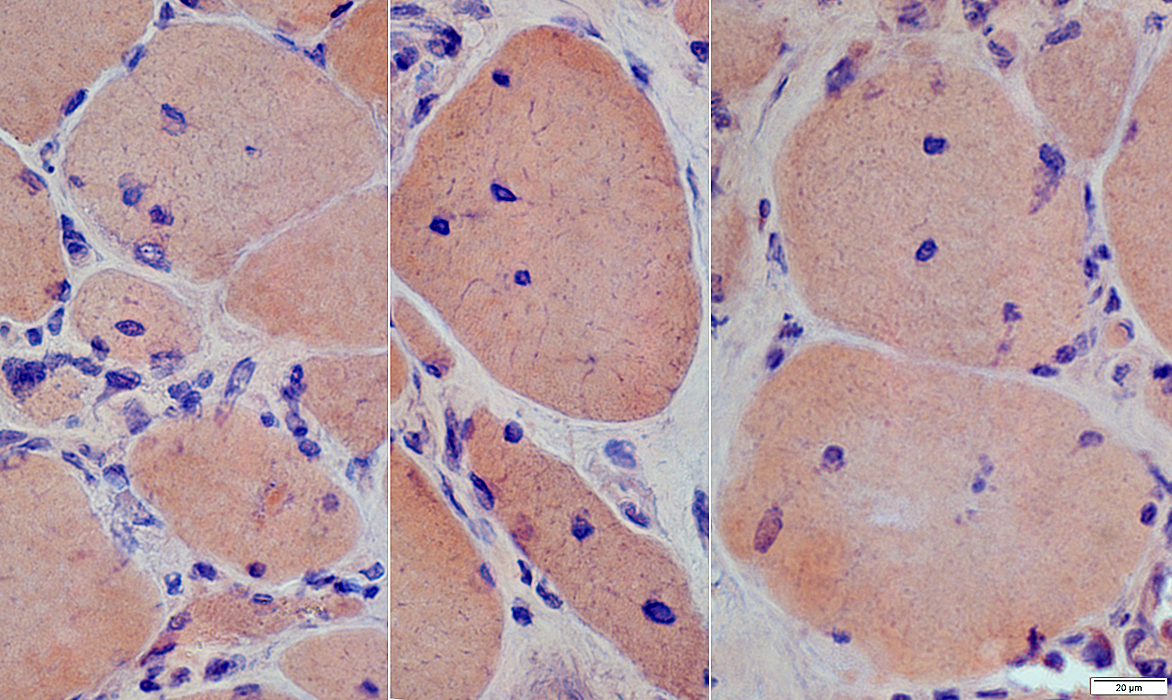
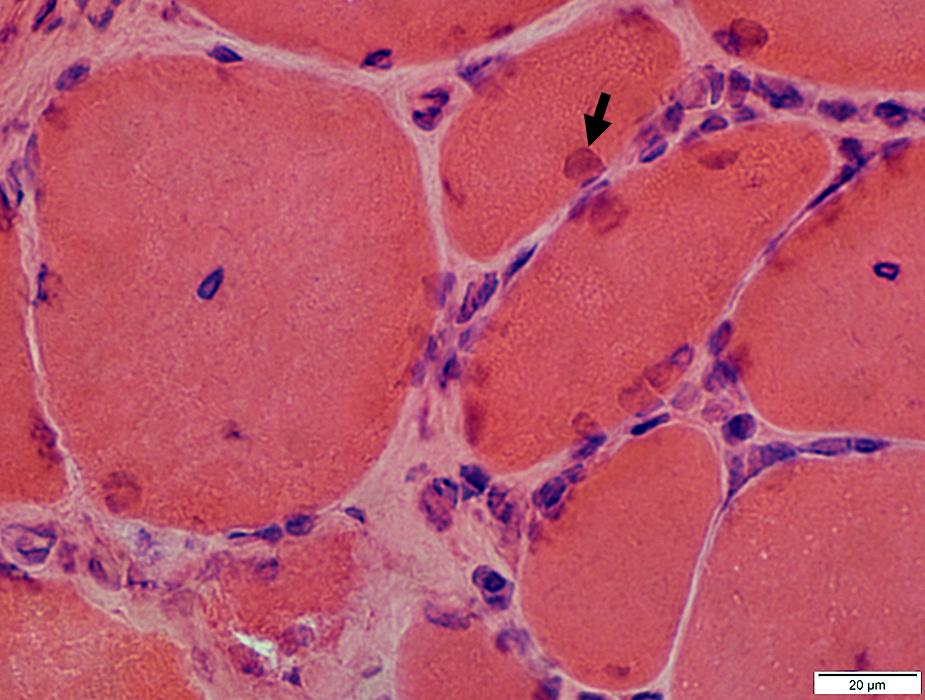 H&E stain |
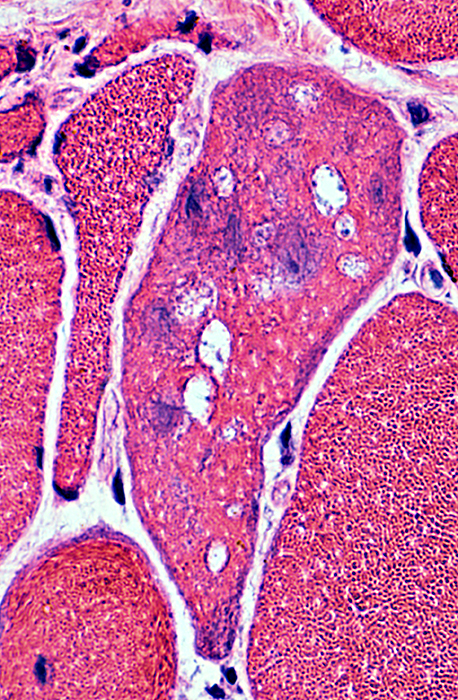 H&E stain |
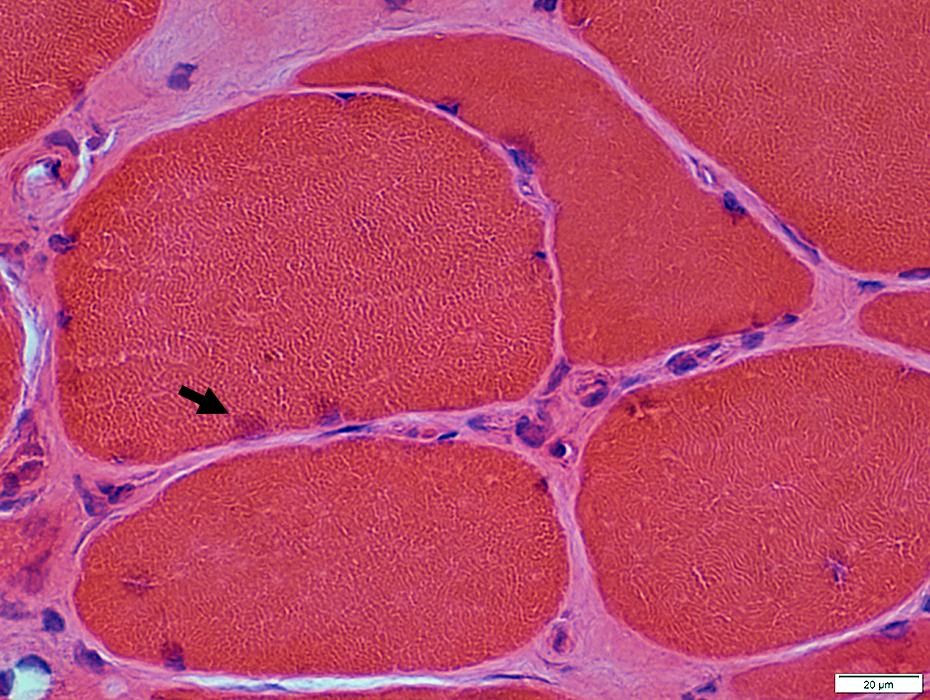 H&E stain |
IM-VAMP Myonuclei: Reduced staining in subsarcolemmal regions of muscle fibers
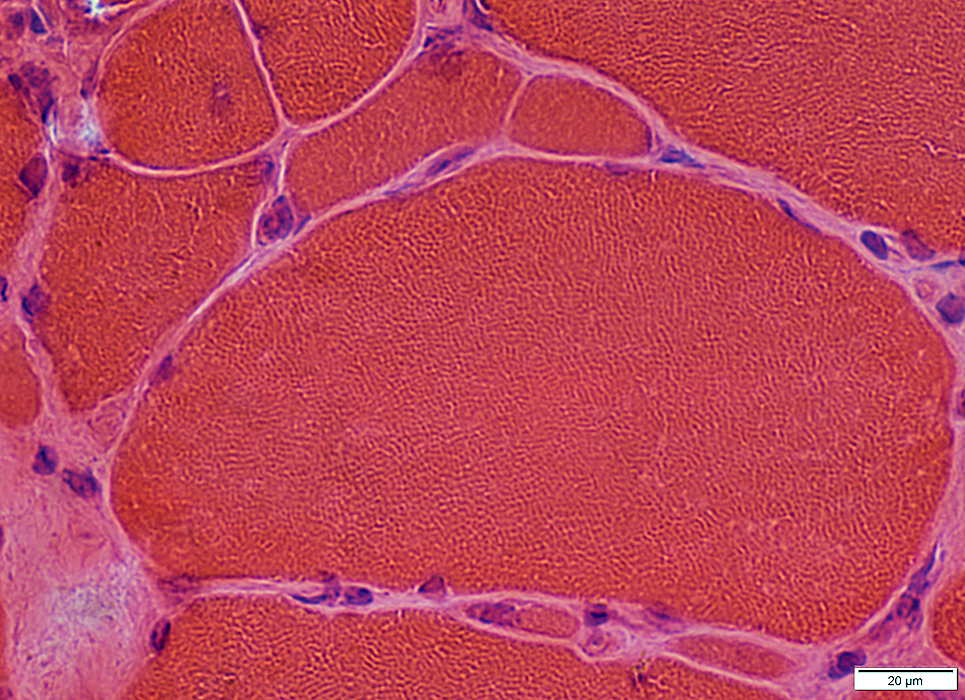 H&E stain |
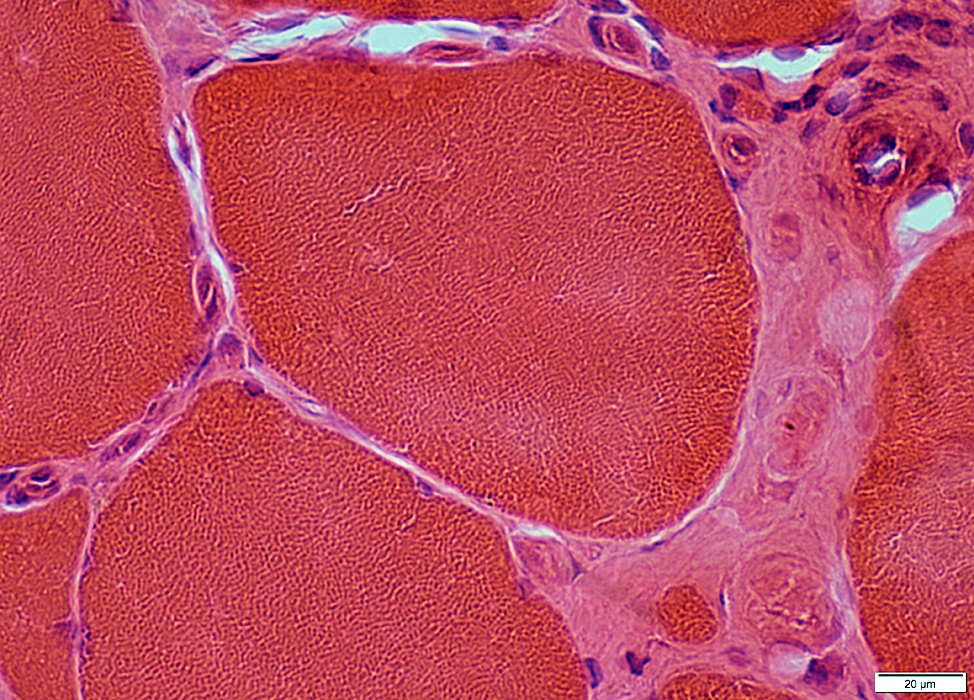 H&E stain |
IM-VAMP (IBM): Mitochondrial Pathology
Molecular PathologyMitochondrial DNA (mtDNA)
Deletions & Duplications 3
Histology
COX- Muscle fibers
SDH+ Muscle fibers
Mitochondrial Ultrastructure
Proliferation
Morphologic Δ
 Cytochrome oxidase stain |
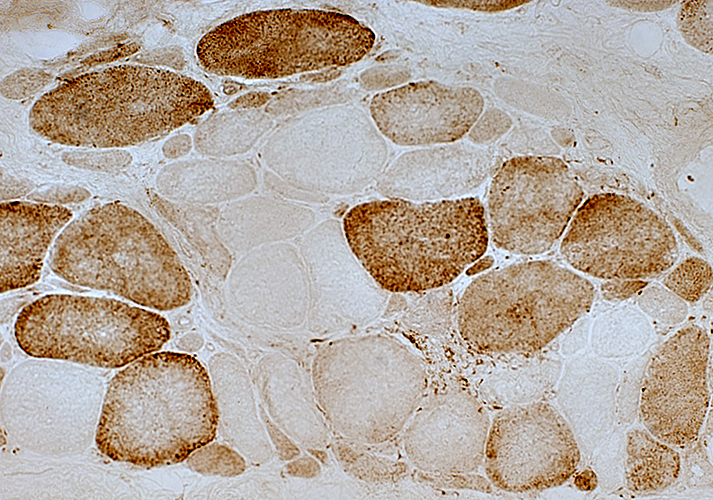 Cytochrome oxidase stain |
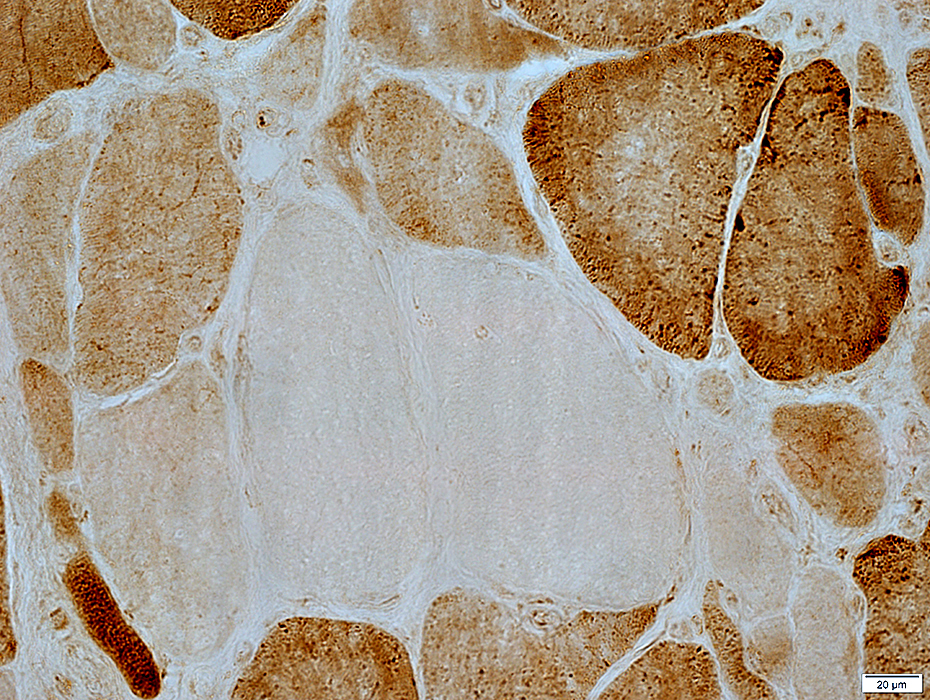 COX stain |
2 muscle fibers with nearly absent staining
Neighboring fibers have normal staining
Changes are likely due to acquired mutations in mtDNA causing loss of synthesis of COX subunits
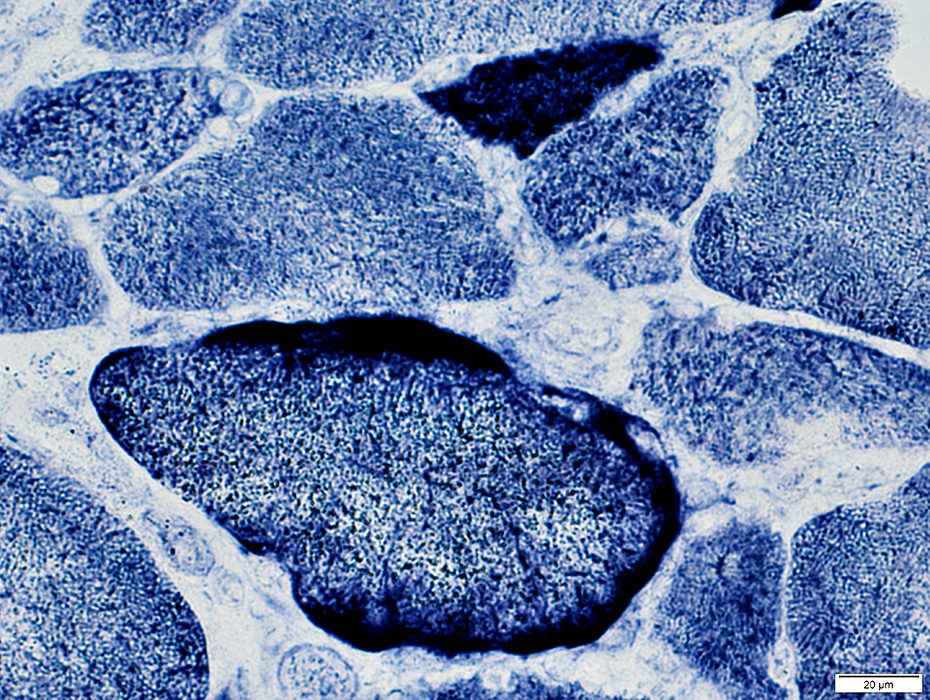 SDH stain |
2 muscle fibers with increased staining
Small muscle fiber above shows increased staining in entire cytoplasm
Larger muscle fiber below has more mitochondrial accumulation in subsarcolemmal regions
Neighboring fibers have normal staining
Changes are likely due to acquired mutations in mtDNA causing mitochondrial proliferation
Also see: Myositis (IM-VAMP) with Mitochondrial Pathology
Ultrastructure
Mitochondrial Pathology in Muscle fibers from this biopsy: SDH stain
 SDH stain |
Ultrastructure: Subsarcolemmal Mitochondrial Accumulation
Also see: Mitochondrial ultrastructure pathology, other
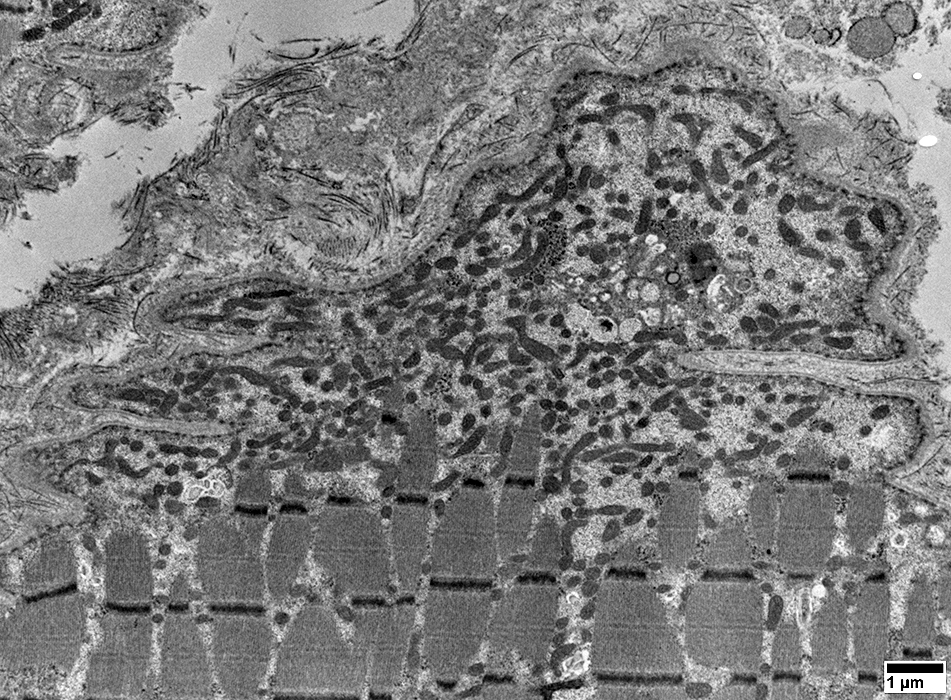 From: R Schmidt |
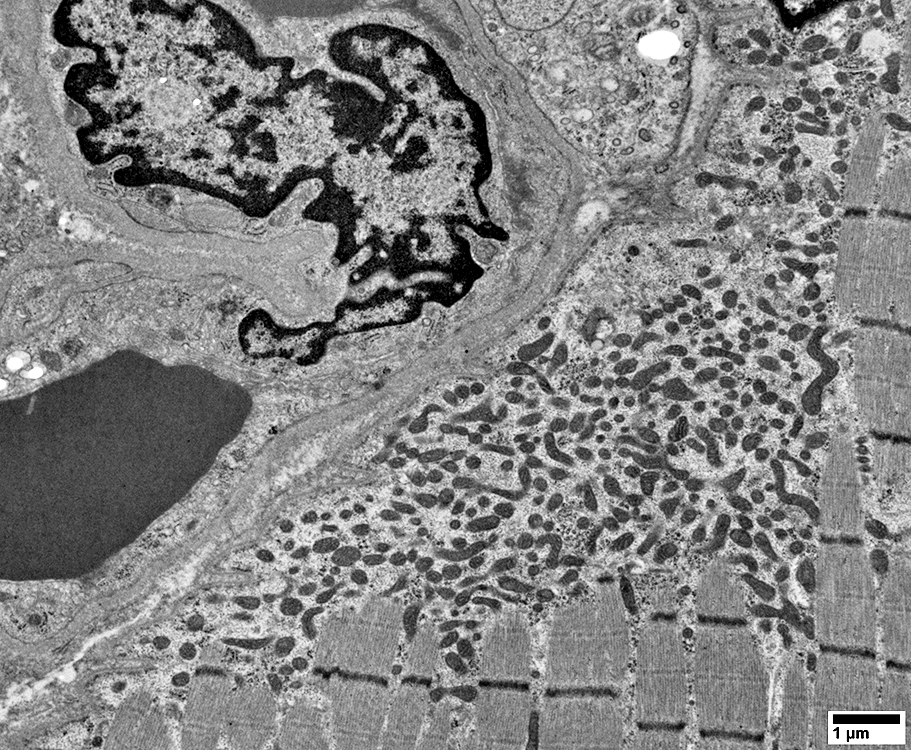 From: R Schmidt |
Mitochondrial pathology: Subsarcolemmal regions
Some areas have dense collections of mitochondria (Left)
Other subsarcolemmal areas have relatively few mitochondria (Right)
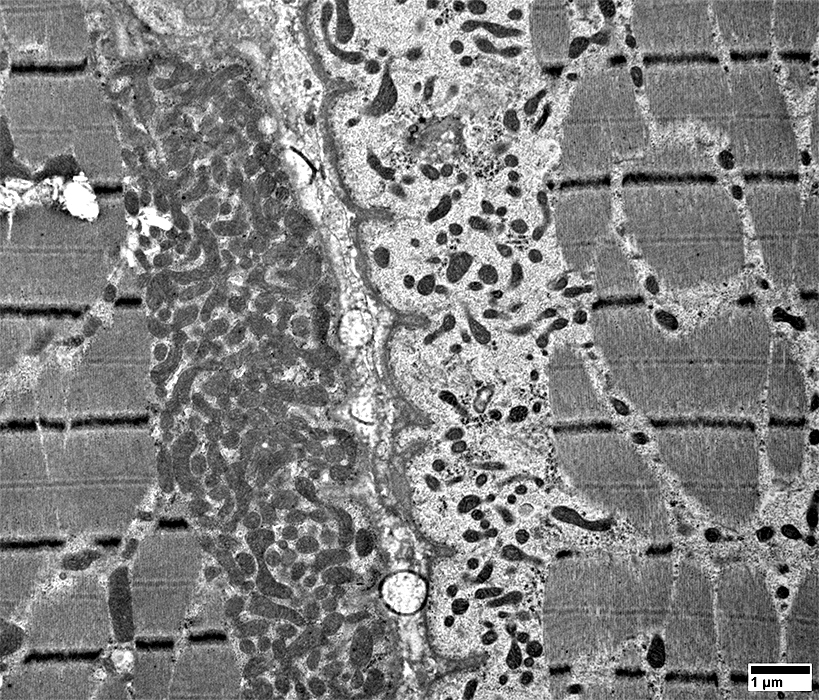 From: R Schmidt |
 From: R Schmidt |
Mitochondria are often
Elongated
Irregular shaped
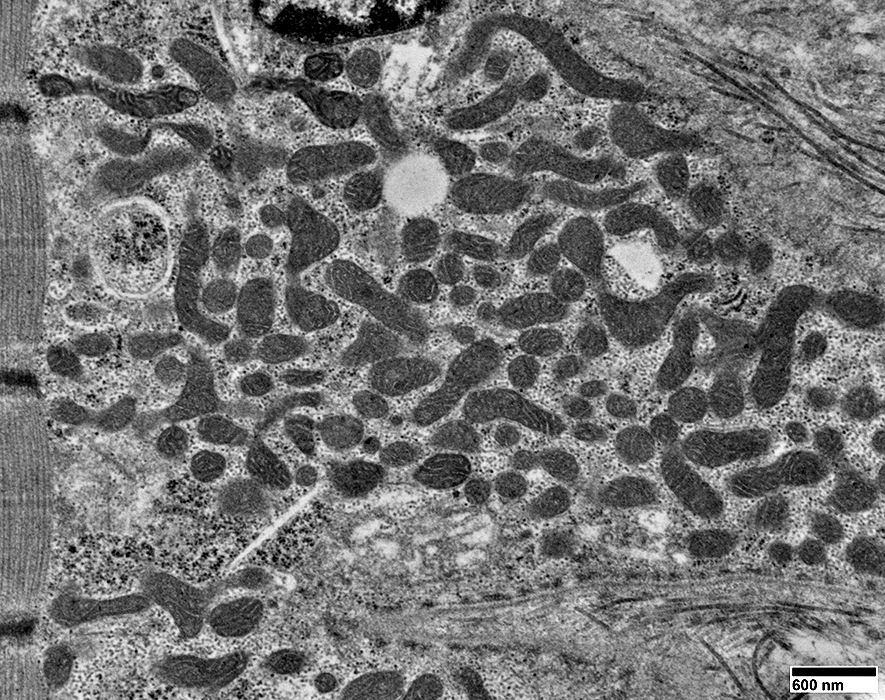
 From: R Schmidt |
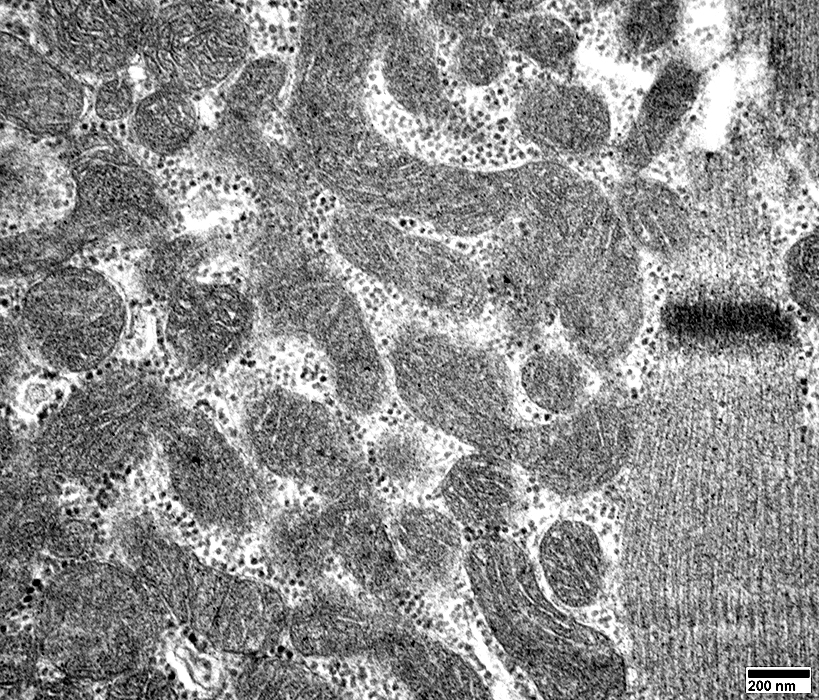 From: R Schmidt |
Mitochondria are often
Elongated
Irregular shaped
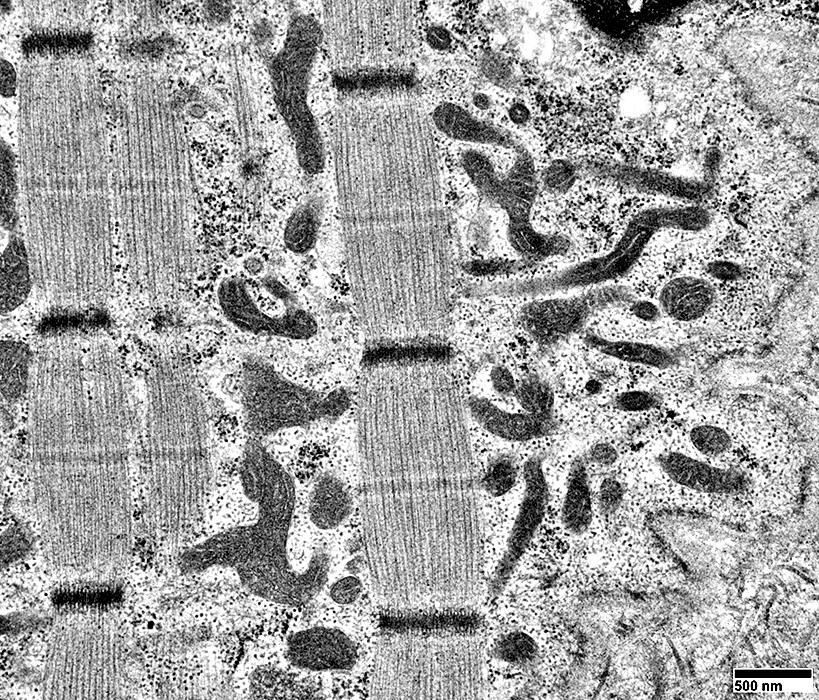
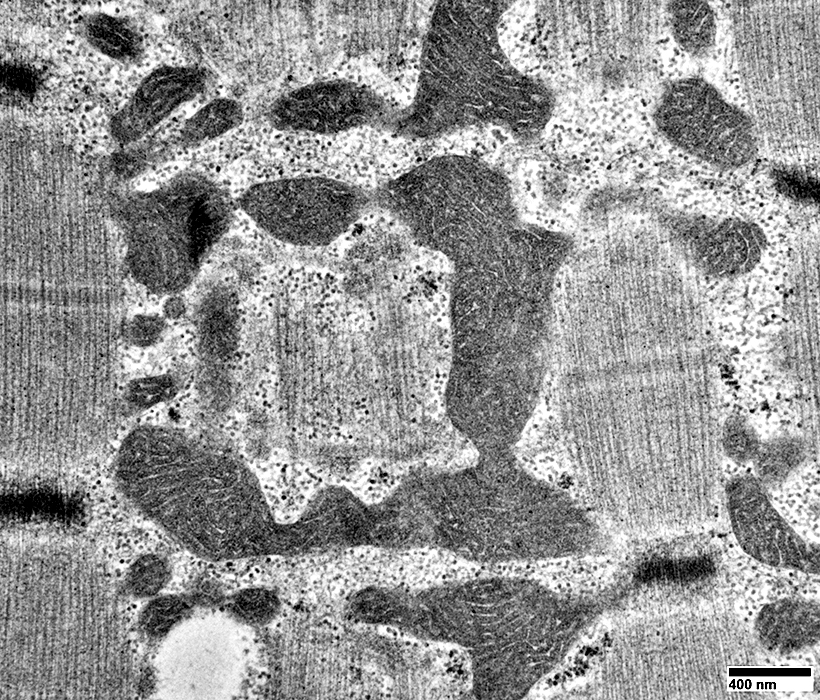 From: R Schmidt |
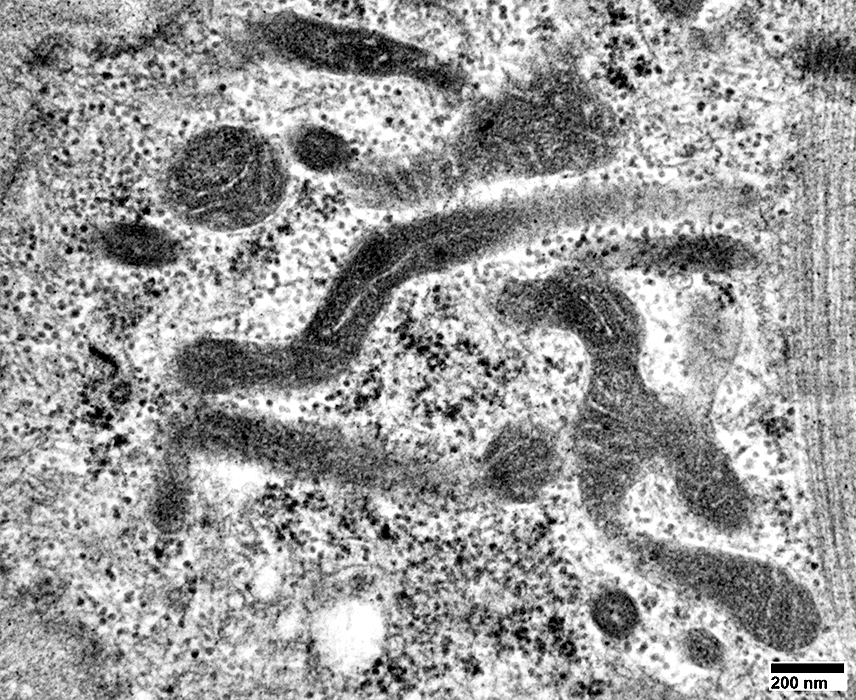 From: R Schmidt |
Mitochondria: Increased Numbers
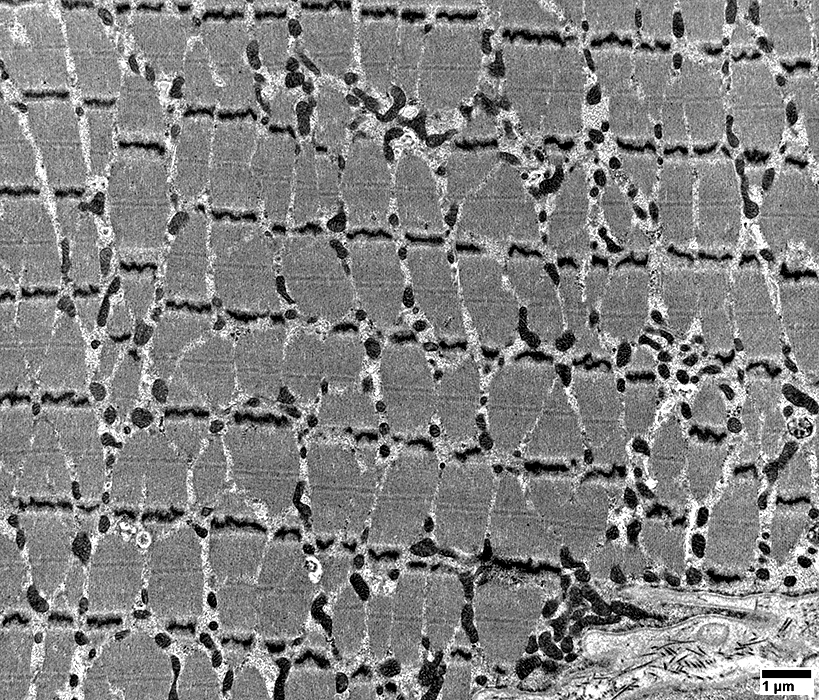 From: R Schmidt |
IBM: Capillary Proliferation & Enlargement
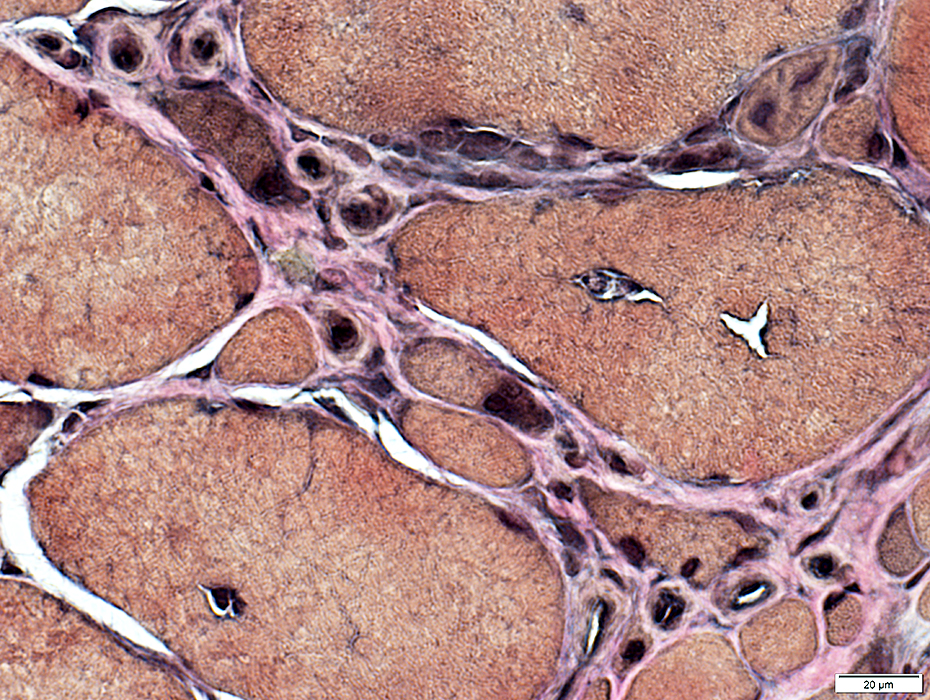 VvG stain |
Large
Numbers: Increased
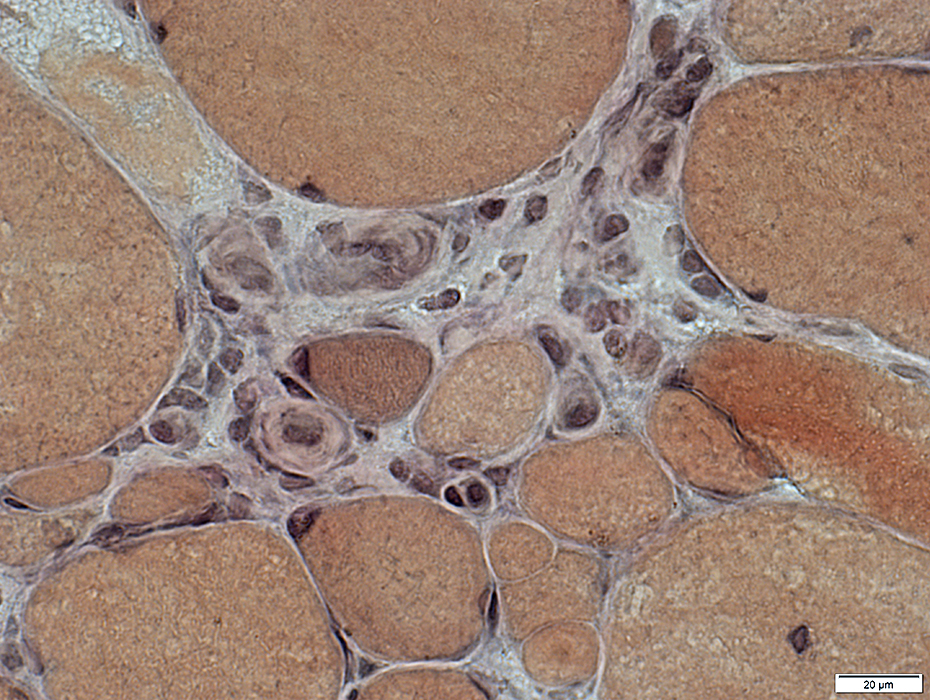 VvG stain |
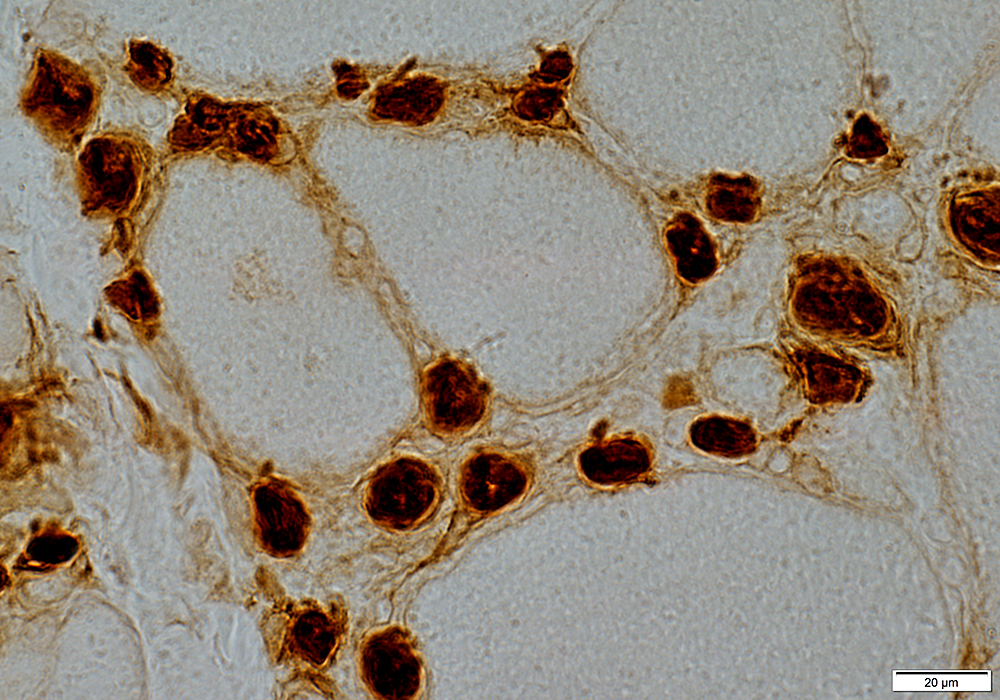 Ulex stain |
Large
Numbers: Increased
Prominent Endothelial Cells
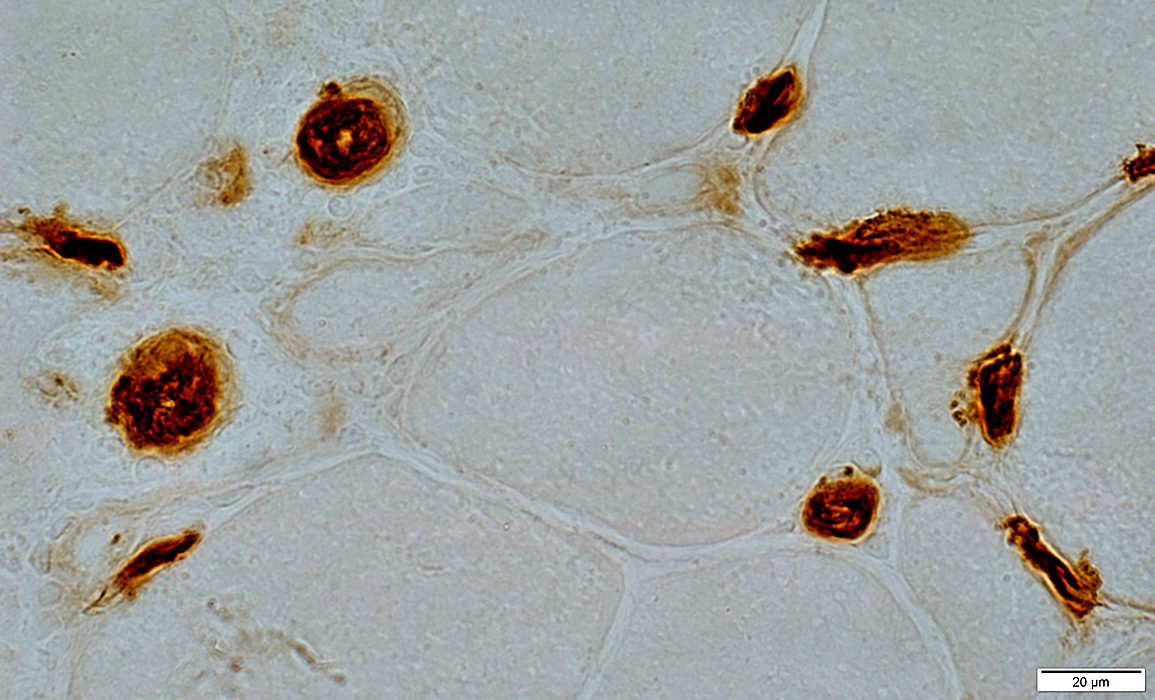 Ulex stain |
Ultrastructure: Cells near Endomysial Capillaries
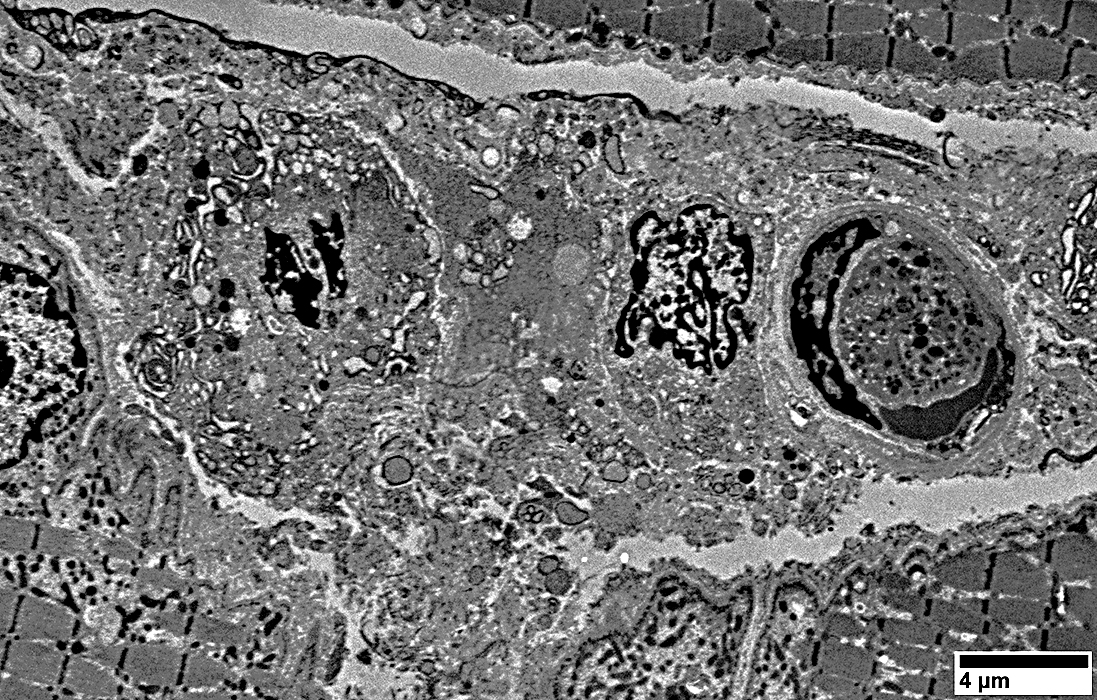 From: R Schmidt |
 From: R Schmidt |
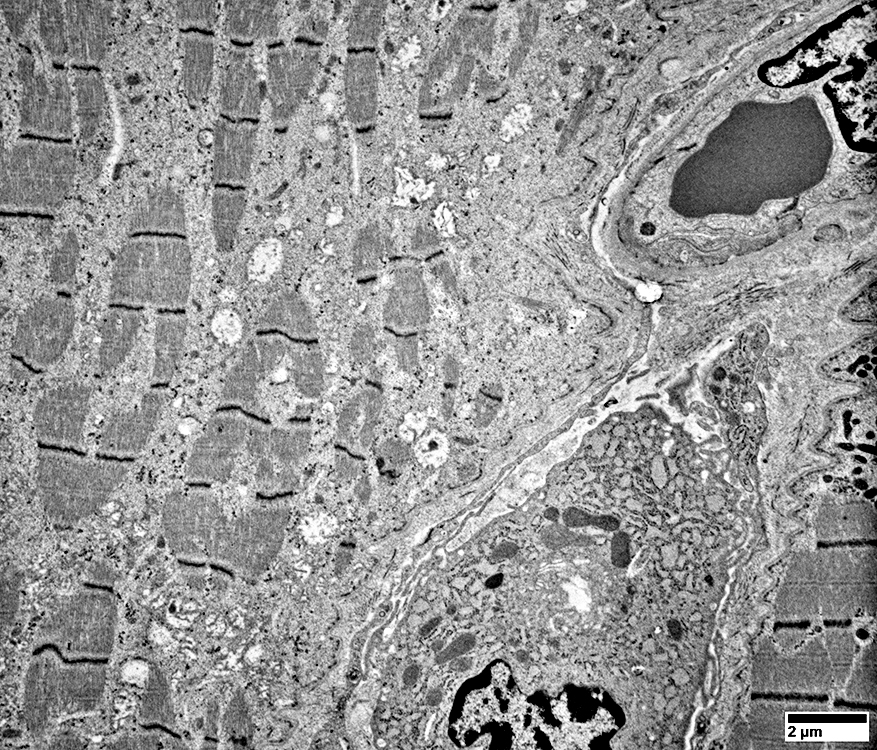 From: R Schmidt |
Return to Neuromuscular Home Page
Return to Inflammation
Return to Inflammatory myopathies & IBM
References
1. Brain 2019 Jul 20
2. Brain 2016;139:1348-1360, Brain 142:2590–2604
3. Brain Pathol 2020 Dec 22;e12931
4. Ann Neurol 2022 Jan 22
5. J Neuropathol Exp Neurol 2022;81:825-835
6. Nat Aging 2024 Jun 4
5/13/2025