Home, Search, Index, Links, Pathology, Molecules, Syndromes,
Muscle, NMJ, Nerve, Spinal, Ataxia, Antibody & Biopsy, Patient Info
|
Home, Search, Index, Links, Pathology, Molecules, Syndromes, Muscle, NMJ, Nerve, Spinal, Ataxia, Antibody & Biopsy, Patient Info |
|
|
| Genes | Syndromes | Inheritance | NM features | Hypermobility | Other |
|
COL5A1
COL5A2 |
Ehlers-Danlos (ED) I |
Dominant |
Hypotonia Fatigue |
Generalized |
Skin: Hyperextensible Bruises; Scars |
| TNXB |
Hypermobility III ED-like + Myopathy |
Dominant Recessive |
Musculoskeletal pain Weakness |
Generalized |
Skin: Bruises; Velvety Hyperextensible; Scars |
|
COL3A1
|
ED 4
|
Dominant |
Tendon & muscle rupture |
Distal |
Skin, Vessel & Organ: Fragility |
|
PLOD
|
ED 6
|
Recessive |
Hypotonia Motor delay |
Distal |
Scoliosis Sclera fragile |
| CHST14 | EDSMC1 | Recessive |
Hypotonia Weakness |
Distal |
Scoliosis |
| DSE | EDSMC2 | Recessive |
Motor delay Weakness |
Distal |
Dysmorphism Sclera: Blue |
|
COL1A1
|
Arthrochalasia (ED VII) |
Dominant |
Hypotonia Motor delay |
Diffuse |
Arthrogryposis Face dysmorphism |
| FKBP14 | EDSKMH | Recessive | Weakness | Distal |
Scoliosis Deafness |
|
FBN1
|
Marfan
|
Dominant |
Muscle atrophy Cramps; Fatigue |
Distal |
Aorta dilatation Arachnodactyly; Scoliosis |
|
TGFBR1
TGFBR2 SMAD3 TGFB2 TGFB3 SMAD2 |
Loeys–Dietz 1
LDS2 LDS3 LDS4 LDS5 LDS6 |
Dominant | Hypotonia | General |
Hypertelorism Aneurysm Contractures |
| COL6A1/A2/A3 |
Ulrich Bethlem |
Recessive Dominant |
Weakness | Distal | Keratosis pilaris |
| COL12A1 |
Ullrich Bethlem |
Recessive Dominant |
Weakness | Distal |
Kyphosis Skin scarring |
| ITGA9 | Ullrich, mild | Recessive |
Weakness Respiratory |
Distal |
Scoliosis |
| SEPN1 | Congenital MD | Recessive |
Weakness Respiratory |
Distal | Rigid spine |
| RYR1 | Mini/Multicore | Recessive | Weakness | Face dysmorphic | |
| CUL4B | Cabezas |
X Recessive |
Muscle wasting |
Short stature Small testes Mental retardation |
|
|
MYH7 Titin |
Multi/Mini-Core | Dominant | Weakness | Distal | |
| β-Sarcoglycan | LGMD 2E | Recessive | Weakness | Distal | |
| ZAK |
Congenital myopathy |
Recessive | Weakness | General |
Scoliosis Type I fiber predominance |
| P4HA1 |
Congenital myopathy |
Recessive | Weakness | Distal |
Contractures Myopia |
|
EMD1: Emerin; Xq28; Recessive EMD2: Lamin A/C; 1q21.2; Dominant EMD3: Lamin A/C; 1q21.2; Recessive EMD4: SYNE1; 6q25; Dominant EMD5: SYNE2; 14q23; Dominant EMD6: FHL1; Xq26; Recessive or Semi-Dominant EMD7; TMEM43; 3p25; Dominant EMD: Titin; 2q31; Recessive EMD modifiers: SUN1; SUN2 Other: Sporadic & Dominant |
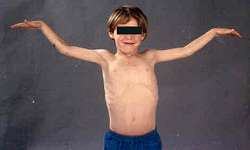 from A Kornberg MD |
|
Genetics Protein Clinical Variant syndromes |
|
|
Syndromes ± Myopathy
|
|
Arthrogryposis: Clinical features
|
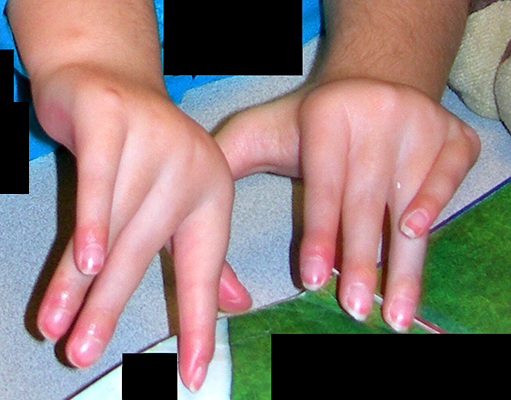
Camptodactyly
|
|
Distal Arthrogryposis Neural molecules 5D: ECEL1; 2q37 5E: PIEZO2; 18p11 Neuropathy Benign CASPR (LCCS7) ADCY6 (LCCS8) GLDN (LCCS11) GPR126 (LCCS9) LGI4 (AMC1) AMCN: ERGIC1 (AMC2) Spinal muscular atrophy Benign congenital 5q-linked, Congenital X-linked Infantile |
|
LCCS (Recessive) 1: GLE1; 9q34 2: ERBB3; 12q13 3: PIP5K1C; 19p13 4: MYBPC1; 12q23 5: DNM2; 19p13 6: ZBTB42; 14q32 7: CNTNAP1; 17q21 8: ADCY6; 12q13 9: GPR126: 6q24 10: NEK9; 14q24 11: Gliomedin; 15q21 LCCS: MAGEL2; 15q11 LCCS: TTN; 2q31 |