PRINCIPLE:
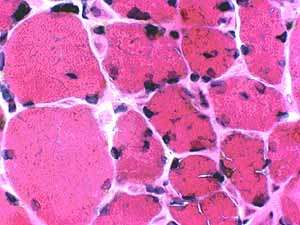 Myopathy with IgM binding to Decorin |
SPECIMEN REQUIRED:
Snap frozen human striated muscle. (Use the
isopentane freezing method previously described.)
METHOD:
Fixation: None,
use snap frozen tissue.
Technique: Cut 10 - 16 micron (12 µm)
sections in cryostat from snap frozen biopsy. Attach one or more sections
to a No.1½, 22 mm square coverslip
- Equipment:
- Ceramic staining rack - Thomas Scientific #8542-E40
- Columbia staining dish - Thomas Scientific #8542-C12
- Columbia staining dish(jar) - Thomas Scientific #8542-E30
- Forceps latex gloves
- Columbia staining dish - Thomas Scientific #8542-C12
- Reagents:
- Reagent alochol - HPLC Fisher A995-4 or histological
A962, FLAMMABLE store at room temp. in a flammable
cabinet
- Eosin Y, disodium salt (Sigma #E-6003, store at room temperature)
- Harris Hematoxylin Stain, acidified (Lerner Laboratories #1931382)(R.T.)
- Permount - Fisher SP15-100, FLAMMABLE HEALTH HAZARD
- Xylenes (Fisher #HC700-1GAL, FLAMMABLE, store R.T. in flammable cabinet)
- Eosin Y, disodium salt (Sigma #E-6003, store at room temperature)
- 1. Eosin Y, 1 % aqueous (store at room temperature)
- Eosin Y dye 1 g
- Deionized water 100 ml
- 2. Harris Hematoxylin, acidified (store at room temperature)
- Filter (Baxter #F2217-150, Grade 363, Qualitative)
before use
- 3. Alcohol 50 %
- Reagent alcohol ~50 ml
- Deionized water ~50 ml
- Deionized water ~50 ml
- 4. Alcohol 70 %
- Reagent alcohol ~70 ml
- Deionized water ~30 ml
- Deionized water ~30 ml
- 5. Alcohol 80 %
- Reagent alcohol ~80 ml
- Deionized water ~20 ml
- Deionized water ~20 ml
- 6. Alcohol 95 %
- Reagent alcohol ~95 ml
- Deionized water ~ 5 ml
Staining Procedure:
1. Place the coverslip with section in a ceramic
staining rack (Thomas Scientific #8542-E40).
2. Immerse sections in the filtered Harris
Hematoxylin for 1 minute.
3. Remove rack to a beaker with tap water.
4. Exchange tap water until the water is clear.
5. Immerse sections in EOSIN stain for 1-2
minutes.
6. Remove rack to a beaker with tap water.
7. Exchange tap water until the water is clear.
8. Dehydrate in ascending alcohol solutions
(50%,70%,80%,95% x 2, 100% x 2) in columbia staining dish(jar)s
- Thomas Scientific #8542-E30 .
9. Clear with xylene (3 - 4 x ) also in columbia staining dish(jar)s - Thomas Scientific #8542-E30.
10. Mount coverslip onto a labeled glass slide
with Permount or some other suitable organic mounting medium.
Results:
Nuclei and other basophilic structures are
blue. Cytoplasm and acidophilic structures are light to dark
red.
REFERENCES:
1. Thompson, Samuel W. SELECTED HISTOCHEMICAL AND HISTOPATHOLOGICAL METHODS, Charles C. Thomas, Springfield, IL, 1966.
2. Sheehan, D.C. and Hrapchak, B.B.: THEORY
AND PRACTICE OF HISTOTECHNOLOGY, 2nd Edition; Battelle Memorial
Institute, Columbus, OH, 1987.
Return to Neuromuscular Home Page
Return to Neuromuscular Evaluations
Return to Muscle biopsy stains
11/19/2002